Draw A Mechanism For This Reaction
Holbox
Mar 29, 2025 · 6 min read
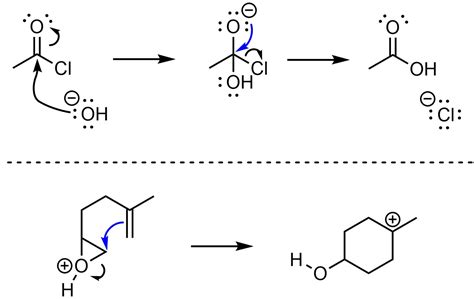
Table of Contents
- Draw A Mechanism For This Reaction
- Table of Contents
- Draw a Mechanism for This Reaction: A Comprehensive Guide
- Understanding the Fundamentals
- Common Reaction Types and Their Mechanisms
- 1. SN1 (Substitution Nucleophilic Unimolecular) Reactions
- 2. SN2 (Substitution Nucleophilic Bimolecular) Reactions
- 3. E1 (Elimination Unimolecular) Reactions
- 4. E2 (Elimination Bimolecular) Reactions
- 5. Addition Reactions
- 6. Nucleophilic Acyl Substitution
- Advanced Concepts and Considerations
- Drawing Effective Mechanisms: A Step-by-Step Approach
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Draw a Mechanism for This Reaction: A Comprehensive Guide
Drawing reaction mechanisms is a cornerstone of organic chemistry. It's not just about memorizing pathways; it's about understanding the why behind chemical transformations. This article provides a comprehensive guide to drawing mechanisms, focusing on a systematic approach that will help you tackle even the most complex reactions. We'll explore fundamental concepts, common reaction types, and provide detailed examples to solidify your understanding.
Understanding the Fundamentals
Before diving into specific reaction mechanisms, let's lay the groundwork. A reaction mechanism is a detailed, step-by-step description of how a reaction occurs at the molecular level. It involves showing the movement of electrons using curved arrows, identifying intermediates, and explaining the changes in bonding and structure.
Key Concepts:
-
Curved Arrows: These are the language of reaction mechanisms. They depict the movement of electron pairs, indicating bond formation (nucleophilic attack) and bond breaking (heterolytic or homolytic cleavage). The arrow's tail starts at the electron source (lone pair or bond), and the head points to the electron sink (atom accepting the electrons).
-
Intermediates: These are short-lived, high-energy species formed during a reaction but not present in the overall stoichiometry. Common intermediates include carbocations, carbanions, radicals, and transition states.
-
Transition States: These are high-energy, unstable structures representing the peak of the activation energy barrier. They are not true intermediates and cannot be isolated. They are often depicted within square brackets with a double dagger symbol (‡).
-
Resonance Structures: Many intermediates can be represented by multiple resonance structures, showing the delocalization of electrons. These structures contribute to the overall stability of the intermediate.
-
Rate-Determining Step: This is the slowest step in the reaction mechanism, determining the overall rate of the reaction.
Common Reaction Types and Their Mechanisms
Let's explore some common reaction types and their associated mechanisms:
1. SN1 (Substitution Nucleophilic Unimolecular) Reactions
SN1 reactions involve a two-step mechanism:
Step 1: Ionization: The leaving group departs, forming a carbocation intermediate. This is the rate-determining step.
(Example: Tertiary alkyl halide reacting with water)
[R3C-X] --> [R3C]+ + X-
Step 2: Nucleophilic Attack: A nucleophile attacks the carbocation, forming a new bond.
[R3C]+ + Nu- --> [R3C-Nu]
Mechanism Illustration: (Imagine a tertiary butyl bromide reacting with water. The bromide leaves, forming a tert-butyl carbocation. A water molecule then attacks the carbocation, forming a tert-butyl alcohol after proton transfer.)
2. SN2 (Substitution Nucleophilic Bimolecular) Reactions
SN2 reactions occur in a single, concerted step:
Mechanism: The nucleophile attacks the substrate from the backside, simultaneously displacing the leaving group. This results in inversion of stereochemistry at the reaction center.
(Example: Methyl bromide reacting with hydroxide ion)
Nu- + [CH3-X] --> [CH3-Nu] + X-
Mechanism Illustration: (Imagine a methyl bromide reacting with hydroxide. The hydroxide attacks the carbon from the backside, pushing out the bromide in one concerted step. The stereochemistry at the carbon inverts if it had chirality).
3. E1 (Elimination Unimolecular) Reactions
E1 reactions are similar to SN1 reactions in that they involve a carbocation intermediate.
Step 1: Ionization: The leaving group departs, forming a carbocation. This is the rate-determining step.
Step 2: Proton Abstraction: A base abstracts a proton from a carbon adjacent to the carbocation, forming a double bond.
(Example: Tertiary alkyl halide reacting with a base)
[R3C-X] --> [R3C]+ + X-
[R3C]+ + B- --> [R2C=CH2] + BH
Mechanism Illustration: (Imagine a tertiary butyl bromide reacting with a base like hydroxide. The bromide leaves, forming a tert-butyl carbocation. The hydroxide then abstracts a proton, forming isobutylene.)
4. E2 (Elimination Bimolecular) Reactions
E2 reactions are concerted, involving a single step where the base abstracts a proton and the leaving group departs simultaneously.
(Example: Alkyl halide reacting with a strong base)
B- + [R-CH2-CH2-X] --> [R-CH=CH2] + BH + X-
Mechanism Illustration: (Imagine ethyl bromide reacting with potassium tert-butoxide. The tert-butoxide abstracts a proton from the beta-carbon while the bromide leaves simultaneously, forming ethene.)
5. Addition Reactions
Addition reactions are common in alkenes and alkynes. The mechanism typically involves the breaking of a pi bond and the formation of two new sigma bonds.
(Example: Electrophilic addition of hydrogen bromide to ethene)
Step 1: Electrophilic Attack: The electrophile (H+) attacks the double bond, forming a carbocation intermediate.
Step 2: Nucleophilic Attack: The nucleophile (Br-) attacks the carbocation, forming the final product.
HBr + CH2=CH2 --> CH3-CH2-Br
Mechanism Illustration: (The proton from HBr adds to one carbon of the double bond, forming a carbocation. The bromide then attacks this carbocation, resulting in bromoethane.)
6. Nucleophilic Acyl Substitution
This reaction type involves the attack of a nucleophile at the carbonyl carbon of an acyl compound (acid chloride, anhydride, ester, amide).
(Example: Hydrolysis of an ester)
Step 1: Nucleophilic Attack: The nucleophile (water) attacks the carbonyl carbon, forming a tetrahedral intermediate.
Step 2: Elimination: The leaving group departs, regenerating the carbonyl group. A proton transfer may be necessary to yield the final product.
Mechanism Illustration: (Water attacks the carbonyl carbon of an ester. A tetrahedral intermediate forms. The alkoxide leaves, followed by a proton transfer, yielding a carboxylic acid and an alcohol).
Advanced Concepts and Considerations
-
Stereochemistry: Pay close attention to stereochemistry throughout the mechanism. SN2 reactions invert stereochemistry, while SN1 and E1 reactions often lead to racemization.
-
Regioselectivity: In reactions like electrophilic addition, consider Markovnikov's rule, which predicts the regioselectivity of the addition.
-
Solvent Effects: The solvent can significantly influence reaction rates and mechanisms. Polar protic solvents favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 reactions.
-
Catalyst Effects: Catalysts can lower the activation energy and influence the reaction pathway.
-
Acid-Base Catalysis: Acids and bases can act as catalysts by protonating or deprotonating reactants, making them more reactive.
Drawing Effective Mechanisms: A Step-by-Step Approach
-
Identify the Reactants and Products: Clearly understand the starting materials and the final products of the reaction.
-
Determine the Reaction Type: Categorize the reaction (SN1, SN2, E1, E2, addition, etc.). This will guide your approach to the mechanism.
-
Identify the Electron-Rich and Electron-Poor Centers: Identify nucleophiles (electron-rich) and electrophiles (electron-poor).
-
Draw the Curved Arrows: Carefully show the movement of electron pairs using curved arrows. Each arrow should represent the movement of two electrons.
-
Draw Intermediates and Transition States: Clearly depict any intermediates and (if appropriate) transition states, keeping in mind their relative stability.
-
Show Proton Transfers: Include proton transfers (acid-base reactions) where necessary to complete the mechanism.
-
Verify the Overall Stoichiometry: Ensure the mechanism accurately reflects the overall stoichiometry of the reaction.
-
Check for Resonance Structures: If applicable, draw resonance structures for intermediates to show electron delocalization.
-
Label the Rate-Determining Step: Identify the slowest step in the mechanism.
Conclusion
Drawing reaction mechanisms is a crucial skill in organic chemistry. By understanding the fundamental principles and following a systematic approach, you can confidently tackle even the most complex reactions. Practice is key – the more mechanisms you draw, the more comfortable you'll become with the process. Remember to always visualize the movement of electrons and the changes in bonding and structure at the molecular level. This approach will not only help you master organic chemistry but also provide a deeper appreciation for the elegance and intricacy of chemical reactions.
Latest Posts
Latest Posts
-
A Government Budget Deficit Exists When
Apr 01, 2025
-
In This Problem A B C And D
Apr 01, 2025
-
People With Lighter Colored Hair Have Melanin In The
Apr 01, 2025
-
Pn Mental Health Online Practice 2023 A
Apr 01, 2025
-
What Is Cold And Comes In Cans
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Draw A Mechanism For This Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
