Diffusion Is One Of The Processes Whereby Materials
Holbox
Mar 29, 2025 · 6 min read
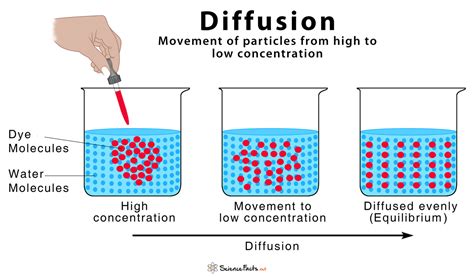
Table of Contents
- Diffusion Is One Of The Processes Whereby Materials
- Table of Contents
- Diffusion: One of the Processes Whereby Materials Move
- Understanding the Basics of Diffusion
- Mechanisms of Diffusion
- 1. Vacancy Diffusion
- 2. Interstitial Diffusion
- 3. Surface Diffusion
- 4. Grain Boundary Diffusion
- 5. Diffusion in Liquids and Gases
- Fick's Laws of Diffusion
- Fick's First Law
- Fick's Second Law
- Factors Affecting Diffusion
- Applications of Diffusion
- 1. Materials Science and Engineering
- 2. Biology and Medicine
- 3. Environmental Science
- 4. Other Applications
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Diffusion: One of the Processes Whereby Materials Move
Diffusion is a fundamental process in nature and engineering, governing the movement of materials from regions of high concentration to regions of low concentration. This seemingly simple phenomenon underpins a vast array of processes, from the transport of nutrients in biological systems to the fabrication of advanced semiconductor devices. Understanding diffusion is crucial in various fields, including materials science, chemistry, biology, and engineering. This article will delve into the intricacies of diffusion, exploring its mechanisms, factors influencing it, and its significant applications.
Understanding the Basics of Diffusion
At its core, diffusion is the net movement of particles (atoms, molecules, or ions) from a region of higher concentration to a region of lower concentration. This movement continues until the particles are evenly distributed throughout the available space, achieving a state of equilibrium. This spontaneous process is driven by the second law of thermodynamics, which dictates that the total entropy (disorder) of a system tends to increase over time. A random distribution of particles represents a higher state of entropy compared to a concentrated one.
Several key aspects define diffusion:
-
Concentration Gradient: The driving force behind diffusion is the concentration gradient, which is the difference in concentration between two regions. A steeper concentration gradient leads to faster diffusion.
-
Flux: The flux represents the rate of diffusion, often expressed as the number of particles passing through a unit area per unit time. It's directly proportional to the concentration gradient (Fick's First Law, discussed later).
-
Equilibrium: Diffusion continues until a state of equilibrium is reached, where the concentration of particles is uniform throughout the system. At equilibrium, the net flux is zero.
Mechanisms of Diffusion
The actual mechanism of diffusion varies depending on the material and the conditions. Several mechanisms exist, including:
1. Vacancy Diffusion
In solids, atoms can move from one lattice site to another if a vacancy (an empty lattice site) is present. This process involves an atom jumping into an adjacent vacancy, leaving behind a new vacancy. The rate of vacancy diffusion depends on the activation energy required for an atom to overcome the energy barrier to jump into the vacancy. Higher temperatures lead to increased atomic vibrations, making it easier for atoms to jump into vacancies and thus increasing the diffusion rate.
2. Interstitial Diffusion
This mechanism involves the movement of smaller atoms (interstitial atoms) through the spaces (interstices) between the atoms of the host lattice. Since interstitial atoms are smaller and don't displace lattice atoms, interstitial diffusion is generally faster than vacancy diffusion. This is commonly seen with small atoms like carbon and hydrogen in metallic matrices.
3. Surface Diffusion
Diffusion can also occur along the surface of a material. Surface diffusion is typically faster than bulk diffusion due to the lower activation energy required for atoms to move on the surface. This mechanism plays a vital role in various surface processes, including catalysis and thin-film growth.
4. Grain Boundary Diffusion
Grain boundaries are regions where the crystal structure of a material is disrupted. Atoms can diffuse more easily along grain boundaries than through the perfect lattice structure of grains. This is because grain boundaries offer lower activation energy paths for diffusion. Grain boundary diffusion is important in polycrystalline materials and influences the overall diffusion behavior.
5. Diffusion in Liquids and Gases
Diffusion in liquids and gases is relatively faster than in solids due to the weaker intermolecular forces. In liquids, molecules move by randomly colliding and exchanging places. In gases, the molecules move independently with high velocities, leading to rapid diffusion.
Fick's Laws of Diffusion
The mathematical description of diffusion is provided by Fick's laws.
Fick's First Law
This law states that the flux (J) is proportional to the concentration gradient (dC/dx):
J = -D (dC/dx)
where:
- J is the diffusion flux
- D is the diffusion coefficient (a measure of how fast diffusion occurs)
- dC/dx is the concentration gradient
The negative sign indicates that diffusion occurs in the direction of decreasing concentration.
Fick's Second Law
This law describes how the concentration changes with time (t) due to diffusion:
∂C/∂t = D (∂²C/∂x²)
This is a partial differential equation that can be solved to predict the concentration profile as a function of time and position.
Factors Affecting Diffusion
Several factors significantly influence the rate of diffusion:
-
Temperature: Higher temperatures increase the kinetic energy of atoms or molecules, leading to faster diffusion. The diffusion coefficient typically follows an Arrhenius-type relationship with temperature.
-
Material Properties: The crystal structure, grain size, and the presence of defects all affect diffusion. Materials with open crystal structures or many defects allow for faster diffusion.
-
Concentration: A steeper concentration gradient results in a faster diffusion rate.
-
Pressure: Pressure can affect the diffusion rate in gases and liquids, though its impact is less significant in solids.
Applications of Diffusion
Diffusion plays a vital role in numerous processes and applications across diverse fields:
1. Materials Science and Engineering
-
Heat Treatment: Diffusion is crucial in heat treatment processes like carburizing (diffusing carbon into steel to increase hardness) and nitriding (diffusing nitrogen to enhance surface properties).
-
Semiconductor Fabrication: Diffusion is essential in the fabrication of integrated circuits, used to introduce dopants into silicon wafers to control electrical conductivity.
-
Powder Metallurgy: Diffusion bonding is a technique where metal powders are sintered together at high temperatures, relying on diffusion to create a strong bond.
-
Alloying: The formation of alloys relies heavily on diffusion processes, as different elements diffuse into each other to create a homogeneous mixture.
2. Biology and Medicine
-
Nutrient Transport: Diffusion is essential for transporting nutrients and oxygen throughout living organisms. For instance, oxygen diffuses from the lungs into the bloodstream and then into cells.
-
Drug Delivery: Diffusion plays a significant role in drug delivery systems, with drugs diffusing through tissues to reach their target sites.
-
Osmosis: Osmosis is a special case of diffusion involving the movement of water molecules across a semipermeable membrane.
3. Environmental Science
- Pollutant Dispersion: Diffusion governs the dispersion of pollutants in the atmosphere and water bodies. Understanding diffusion is crucial for environmental modeling and remediation efforts.
4. Other Applications
-
Gas Separation: Diffusion is used in various gas separation processes, such as separating isotopes.
-
Electroplating: The deposition of metals during electroplating is also based on diffusion.
Conclusion
Diffusion is a ubiquitous process with far-reaching implications across various scientific and engineering disciplines. Its understanding is essential for controlling and manipulating material properties, designing efficient processes, and developing new technologies. The principles of diffusion, described by Fick's laws, provide a powerful framework for analyzing and predicting the movement of materials. Continued research and development in this area will continue to unlock further applications and refine our understanding of this fundamental process. The complexity of diffusion, with its various mechanisms and influencing factors, offers endless opportunities for innovation and discovery. From the microscopic scale of atomic movement to the macroscopic scale of environmental processes, diffusion remains a fascinating and critical aspect of our world. Further exploration of advanced techniques and mathematical models will further enhance our capability to harness the power of diffusion for the betterment of science and technology.
Latest Posts
Latest Posts
-
You Receive An Email Marked Important From Your Boss
Apr 02, 2025
-
The Bureau Of Transportation Statistics Collects Analyzes And Disseminates
Apr 02, 2025
-
Stirring The Mixture Does Which Of The Following Select Two
Apr 02, 2025
-
Comity Is A Doctrine That Is Rooted In
Apr 02, 2025
-
What Material Makes Up Most Of The Structure At A
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Diffusion Is One Of The Processes Whereby Materials . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
