Density Of Mercury In Kg M3
Holbox
Mar 11, 2025 · 5 min read
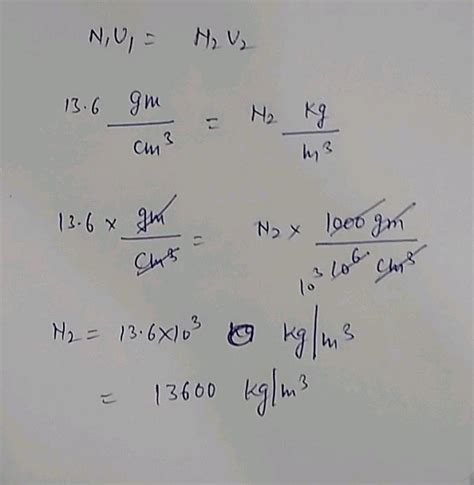
Table of Contents
- Density Of Mercury In Kg M3
- Table of Contents
- Density of Mercury in kg/m³: A Comprehensive Guide
- What is Density?
- The Density of Mercury: A Deep Dive
- Factors Affecting Mercury Density
- Measurement of Mercury Density
- Significance of Mercury's Density
- Safety Precautions when Handling Mercury
- Environmental Concerns Related to Mercury
- Conclusion
- Latest Posts
- Related Post
Density of Mercury in kg/m³: A Comprehensive Guide
Mercury, a fascinating and unique element, holds a significant place in various scientific and industrial applications. Understanding its properties, particularly its density, is crucial for numerous applications, from scientific research to industrial processes. This comprehensive guide delves into the density of mercury in kg/m³, exploring its measurement, variations, significance, and applications. We'll also delve into the safety precautions necessary when handling this dense, toxic liquid metal.
What is Density?
Before we delve into the specifics of mercury's density, let's establish a clear understanding of the concept itself. Density is a fundamental physical property of matter defined as the mass per unit volume. It essentially tells us how much "stuff" is packed into a given space. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
The standard unit for density is kilograms per cubic meter (kg/m³), although other units like grams per cubic centimeter (g/cm³) are also commonly used. One gram per cubic centimeter is equivalent to 1000 kg/m³.
The Density of Mercury: A Deep Dive
Mercury, with its chemical symbol Hg (from the Latin hydrargyrum, meaning "liquid silver"), is the only metallic element that exists as a liquid at standard temperature and pressure. This unique property, along with its high density, contributes to its wide range of applications.
The density of mercury at 0°C (273.15 K) is approximately 13,534 kg/m³. This exceptionally high density is a result of mercury's high atomic mass and its relatively close atomic packing in its liquid state. It's roughly 13.6 times denser than water, which has a density of approximately 1000 kg/m³ at 4°C.
Factors Affecting Mercury Density
While the density of mercury at standard conditions is relatively constant, several factors can subtly influence its value:
-
Temperature: Density is inversely proportional to temperature. As temperature increases, the volume of mercury expands, leading to a decrease in density. This effect is relatively small, but it's crucial for precise measurements. Accurate density determination requires careful temperature control. Corrections must be made when using density values at different temperatures.
-
Pressure: Pressure also plays a minor role in influencing mercury's density. Increased pressure leads to a slight compression of the mercury, resulting in a marginally higher density. However, this effect is typically negligible at pressures commonly encountered.
-
Impurities: The presence of impurities in the mercury sample can alter its density. If other substances are dissolved or suspended within the mercury, it will affect the overall mass and volume, thus altering the density. High-purity mercury is crucial for accurate density measurements in scientific applications.
Measurement of Mercury Density
Precise determination of mercury density involves careful experimental techniques. Several methods can be employed:
-
Pycnometry: This classical method involves determining the mass and volume of a mercury sample using a precisely calibrated pycnometer (a specialized density bottle). This method offers high accuracy, especially when combined with careful temperature control and correction for buoyancy effects.
-
Hydrostatic Weighing: This method uses Archimedes' principle to measure the buoyant force on a submerged object. By carefully measuring the apparent weight of a sinker in mercury and in a fluid of known density (like water), the density of mercury can be calculated. This technique is relatively simple but requires careful calibration and correction for buoyancy effects.
-
X-ray Diffraction: While less directly used for density measurement, X-ray diffraction techniques can provide information about the atomic arrangement within the mercury, which in turn provides insights into the density at the atomic level. This method is more suited for fundamental research rather than routine density measurements.
Significance of Mercury's Density
The high density of mercury has several crucial implications across various fields:
-
Scientific Applications: Mercury's high density makes it useful in various scientific instruments, such as barometers (measuring atmospheric pressure) and manometers (measuring pressure differences). Its high density allows for compact designs and sensitive readings.
-
Industrial Applications: High-density liquids are needed for certain industrial processes. Mercury's density is utilized in applications like separating minerals using gravity separation techniques. However, environmental concerns are increasingly limiting these applications.
-
Medical Applications (Historical): Although largely discontinued due to its toxicity, mercury's density and other properties were previously used in certain medical applications like thermometers. These applications are now considered obsolete.
-
Amalgams: Mercury's ability to readily form amalgams (alloys with other metals) is partly linked to its density. The density of the resulting amalgam will depend on the ratio of mercury to other metals.
Safety Precautions when Handling Mercury
It's critical to emphasize the extreme toxicity of mercury and the importance of rigorous safety precautions when handling it:
-
Avoid Direct Contact: Mercury is readily absorbed through skin, inhalation, and ingestion. Any direct contact should be avoided. Protective gear, including gloves and eye protection, must always be worn.
-
Ventilation: Mercury vapor is highly toxic. Adequate ventilation is crucial to prevent inhalation of mercury vapor, especially in enclosed spaces where mercury is being handled.
-
Spill Response: In case of a mercury spill, immediate action is essential. Specialized mercury spill kits should be used to safely contain and clean up the spilled mercury.
-
Disposal: Disposal of mercury and mercury-containing materials should adhere to local and national environmental regulations. Improper disposal can cause significant environmental contamination.
Environmental Concerns Related to Mercury
The high density of mercury contributes to its persistence in the environment. Spilled mercury can settle into the sediment of waterways and remain there for extended periods, posing a long-term environmental threat. The environmental impacts of mercury necessitate strict regulations and responsible handling procedures. Bioaccumulation of mercury in the food chain is a major concern.
Conclusion
The density of mercury, approximately 13,534 kg/m³ at 0°C, is a defining characteristic that underlies many of its properties and applications. While its high density provides certain advantages in scientific instruments and industrial processes, the extreme toxicity of mercury necessitates rigorous safety protocols and environmentally conscious handling. Understanding its density and associated safety concerns is crucial for anyone working with or around this unique element. The responsible use and disposal of mercury are paramount to mitigating environmental and health risks. Future advancements will likely focus on finding safer alternatives for applications previously reliant on mercury's unique properties. The continuing research into its behavior and impact on the environment underscores its significant importance and the need for ongoing vigilance.
Latest Posts
Related Post
Thank you for visiting our website which covers about Density Of Mercury In Kg M3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.