Consider The Cyclohexane Framework In A Chair Conformation
Holbox
Mar 16, 2025 · 5 min read
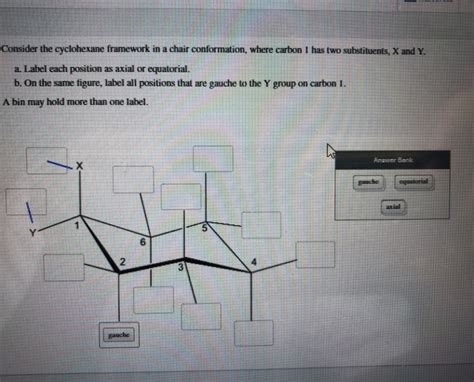
Table of Contents
Consider the Cyclohexane Framework in a Chair Conformation
Cyclohexane, a seemingly simple molecule (C₆H₁₂), presents a fascinating case study in organic chemistry, primarily due to its conformational flexibility. Understanding its chair conformation is crucial for grasping the principles of stereochemistry and the reactivity of many larger, more complex molecules. This article delves deep into the cyclohexane chair conformation, exploring its stability, substituent effects, and implications for organic reactions.
The Chair Conformation: Stability and Energy Minimization
Cyclohexane doesn't exist as a flat, planar hexagon. Such a structure would force significant angle strain (deviation from the ideal 109.5° bond angle of sp³ hybridized carbon) and eclipsing interactions between hydrogen atoms. To minimize these destabilizing factors, cyclohexane adopts a chair conformation.
Key Features of the Chair Conformation:
- Staggered Conformation: All adjacent C-H bonds are staggered, minimizing torsional strain. This arrangement is significantly more stable than the eclipsed conformation.
- Optimal Bond Angles: The chair conformation allows for bond angles very close to the ideal tetrahedral angle of 109.5°, minimizing angle strain.
- Axial and Equatorial Positions: Each carbon atom in the chair conformation has two distinct positions for substituents: axial and equatorial. Axial bonds are parallel to the axis of symmetry of the ring, while equatorial bonds are roughly perpendicular. This distinction is crucial when considering the steric effects of substituents.
Energy Differences Between Conformations:
While the chair conformation is the most stable, cyclohexane can also adopt other less stable conformations, such as the boat and twist-boat conformations. The boat conformation suffers from significant steric interactions between the flagpole hydrogens, making it significantly higher in energy. The twist-boat conformation represents a transition state between two chair conformations, slightly lower in energy than the boat but still substantially less stable than the chair. The energy difference between the chair conformation and these other conformations is significant enough that at room temperature, the vast majority of cyclohexane molecules exist in the chair conformation.
Substituent Effects on Chair Conformation:
Introducing a substituent to the cyclohexane ring introduces a new layer of complexity. The stability of the chair conformation now depends on the size and steric bulk of the substituent.
1,3-Diaxial Interactions:
The crucial factor influencing the stability of substituted cyclohexane is 1,3-diaxial interactions. These occur when an axial substituent interacts sterically with axial hydrogens (or other substituents) on the 1,3-carbons. Larger substituents lead to stronger 1,3-diaxial interactions, destabilizing the chair conformation with the substituent in the axial position.
Equitorial vs. Axial:
As a result, substituted cyclohexanes overwhelmingly prefer the conformation with the largest substituents in the equatorial position. This minimizes 1,3-diaxial interactions and maximizes overall stability. For instance, methylcyclohexane strongly prefers the conformation with the methyl group equatorial, which is more stable by approximately 7.6 kJ/mol than the conformation with the methyl group axial. This energy difference increases with the size of the substituent. Bulky tert-butyl groups, for example, almost exclusively reside in the equatorial position.
Conformational Equilibrium:
When a substituent is present, the cyclohexane ring interconverts between two chair conformations—one with the substituent axial and the other with the substituent equatorial. This interconversion occurs via a rapid ring flip. The equilibrium between these two conformations is governed by the relative energies of each conformer, with the equatorial conformer predominating for larger substituents. The equilibrium constant K is directly related to the energy difference (ΔG°) between the two conformations through the equation: ΔG° = -RTlnK.
Anomeric Effect: A Special Case
The anomeric effect is a deviation from the typical preference for equatorial substituents. It is observed in cyclic acetals and glycosides, where an electronegative substituent (like an oxygen atom) bonded to a carbon adjacent to a ring oxygen displays a preference for the axial position. This is due to the stabilization gained by hyperconjugative interactions between the lone pair electrons of the electronegative atom and the antibonding σ* orbital of the C-O bond.
Implications for Organic Reactions:
The chair conformation of cyclohexane and its substituent effects profoundly influence the outcome of many organic reactions. Understanding these conformational preferences is essential for predicting reaction rates, regioselectivity, and stereoselectivity.
Reactivity of Axial vs. Equatorial Substituents:
Axial substituents are generally more reactive than equatorial substituents due to increased steric accessibility to attacking reagents. This difference in reactivity can be exploited to achieve selective reactions.
Stereoselective Reactions:
Many reactions involving cyclohexane derivatives exhibit stereoselectivity—a preference for one stereoisomer over another. This stereoselectivity is often controlled by the conformational preferences of the starting materials. For instance, the reaction of a substituted cyclohexane with a reagent might selectively occur from the less hindered side, leading to a specific stereoisomer as the major product.
Conformational Analysis in Complex Molecules:
The principles of conformational analysis established with cyclohexane are readily extended to more complex cyclic systems, aiding in predicting their three-dimensional structures and reactivities. This knowledge is essential in designing and synthesizing molecules with specific properties and functionalities.
Analyzing Complex Systems: Beyond Monosubstituted Cyclohexanes
While the principles discussed above largely pertain to monosubstituted cyclohexanes, the complexity increases significantly with polysubstituted cyclohexanes. Multiple substituents introduce intricate interplay between 1,3-diaxial interactions and the overall steric profile of the molecule. Predicting the most stable conformation becomes more challenging, often requiring more sophisticated computational tools and analysis techniques.
Computational Methods and Visualization:
Modern computational chemistry techniques play a vital role in analyzing the conformations of cyclohexane derivatives. Molecular mechanics and density functional theory calculations can accurately predict the relative energies of various conformations, providing insights into the most stable arrangement and the energy barriers involved in conformational changes. Molecular visualization software further aids in understanding the spatial relationships between atoms and substituents within the molecule, providing a clearer picture of the steric interactions at play.
Conclusion:
The chair conformation of cyclohexane is a cornerstone of organic chemistry. Understanding its stability, the effects of substituents, and its implications for reactivity is crucial for anyone studying organic molecules. The interplay between conformational analysis and reaction mechanisms provides a powerful framework for designing organic syntheses and predicting the behavior of molecules in chemical reactions. Furthermore, the extension of these principles to more complex systems highlights the importance of conformational analysis in addressing sophisticated chemical problems. The ongoing development and refinement of computational techniques further enhances our ability to explore and understand the complex world of molecular conformations, constantly enriching our understanding of organic chemistry.
Latest Posts
Latest Posts
-
Draw The Missing Organic Structures Do Not Draw Inorganic By Products
Mar 17, 2025
-
National Party Organizations Can Dictate The Day To Day Decisions Of
Mar 17, 2025
-
Dealing With Difficult Clients Negatively Impacts My Disposition
Mar 17, 2025
-
Your Company Offers A Single Premium Mobile Phone Handset
Mar 17, 2025
-
Which Of The Following Is True Select All That Apply
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Consider The Cyclohexane Framework In A Chair Conformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
