Consider The Acid Catalyzed Hydration Of 3 Methyl 1 Butene
Holbox
Mar 28, 2025 · 6 min read
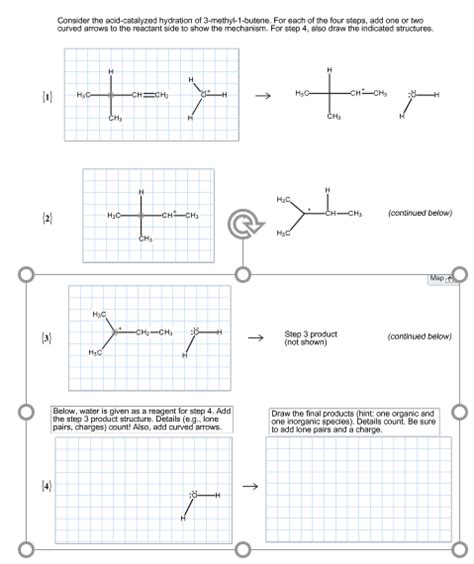
Table of Contents
- Consider The Acid Catalyzed Hydration Of 3 Methyl 1 Butene
- Table of Contents
- Consider the Acid-Catalyzed Hydration of 3-Methyl-1-butene
- Understanding Markovnikov's Rule and its Application to 3-Methyl-1-butene
- The Detailed Mechanism of Acid-Catalyzed Hydration
- Step 1: Protonation of the Alkene
- Step 2: Nucleophilic Attack by Water
- Step 3: Deprotonation
- Regioselectivity and Stereochemistry
- Reaction Conditions and Optimization
- Potential Side Reactions
- Comparison with Other Hydration Methods
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Consider the Acid-Catalyzed Hydration of 3-Methyl-1-butene
The acid-catalyzed hydration of alkenes is a fundamental reaction in organic chemistry, offering a versatile method for synthesizing alcohols. This process involves the addition of water across the carbon-carbon double bond, with the hydroxyl group (-OH) attaching to the more substituted carbon atom according to Markovnikov's rule. This article delves into the detailed mechanism of the acid-catalyzed hydration of 3-methyl-1-butene, exploring the intricacies of the reaction, potential side reactions, regioselectivity, and the importance of reaction conditions.
Understanding Markovnikov's Rule and its Application to 3-Methyl-1-butene
Before delving into the mechanism, it's crucial to understand Markovnikov's rule. This rule dictates that in the addition of a protic acid (HX) to an unsymmetrical alkene, the hydrogen atom (H) bonds to the carbon atom that already possesses more hydrogen atoms, while the X group (in this case, the -OH group) attaches to the carbon atom with fewer hydrogen atoms. This results in the formation of the more stable carbocation intermediate.
Applying this to 3-methyl-1-butene, the reaction with water in the presence of an acid catalyst will yield predominantly 3-methyl-2-butanol. This is because the protonation of the double bond leads to the formation of a more stable secondary carbocation rather than a less stable primary carbocation. The secondary carbocation is more stable due to the inductive effect of the adjacent methyl groups, which donate electron density to stabilize the positive charge.
The Detailed Mechanism of Acid-Catalyzed Hydration
The acid-catalyzed hydration of 3-methyl-1-butene proceeds through a three-step mechanism:
Step 1: Protonation of the Alkene
The reaction begins with the protonation of the alkene's double bond by a strong acid catalyst, such as sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄). The electrophilic proton attacks the less substituted carbon atom, generating a more stable secondary carbocation intermediate. This step is reversible, and the equilibrium lies towards the protonated alkene due to the relative stability of the carbocation formed.
Reaction:
3-Methyl-1-butene + H⁺ ⇌ 3-Methyl-2-butyl carbocation
Step 2: Nucleophilic Attack by Water
In the second step, a water molecule acts as a nucleophile, attacking the electrophilic carbocation. The oxygen atom of the water molecule forms a bond with the positively charged carbon atom, creating an oxonium ion intermediate. This step is relatively fast, as the positively charged carbocation is highly reactive.
Reaction:
3-Methyl-2-butyl carbocation + H₂O → 3-Methyl-2-butanol oxonium ion
Step 3: Deprotonation
The final step involves the deprotonation of the oxonium ion intermediate. A water molecule acts as a base, abstracting a proton from the oxonium ion, resulting in the formation of 3-methyl-2-butanol and regenerating the acid catalyst. This step is also relatively fast.
Reaction:
3-Methyl-2-butanol oxonium ion + H₂O → 3-Methyl-2-butanol + H₃O⁺
Regioselectivity and Stereochemistry
The acid-catalyzed hydration of 3-methyl-1-butene exhibits high regioselectivity, favoring the formation of 3-methyl-2-butanol. This is a direct consequence of Markovnikov's rule, which predicts the addition of the hydroxyl group to the more substituted carbon atom. The formation of the more stable secondary carbocation intermediate is the driving force behind this regioselectivity.
Regarding stereochemistry, the reaction is not stereospecific. The carbocation intermediate is planar, and the nucleophilic attack by water can occur from either side of the plane, leading to a racemic mixture of (R)- and (S)-3-methyl-2-butanol. This means the product will be a 50:50 mixture of both enantiomers, lacking any significant enantiomeric excess.
Reaction Conditions and Optimization
The success of the acid-catalyzed hydration reaction depends significantly on the reaction conditions. Several factors can influence the yield and selectivity of the reaction:
-
Acid Catalyst: The choice of acid catalyst is crucial. Strong acids like sulfuric acid and phosphoric acid are commonly employed. The concentration of the acid catalyst also plays a role, with higher concentrations generally leading to faster reaction rates, but potentially increased side reactions.
-
Temperature: The reaction temperature is another important factor. Higher temperatures can accelerate the reaction rate but may also promote side reactions, such as alkene isomerization or dehydration. A moderate temperature is often preferred to optimize yield and minimize side reactions.
-
Water Concentration: Sufficient water concentration is necessary for the reaction to proceed efficiently. The use of excess water ensures that the water acts as both a reactant and a solvent, facilitating the reaction.
-
Reaction Time: The reaction time must be optimized to achieve high conversion without excessive side reactions. Monitoring the progress of the reaction is crucial to determine the optimal reaction time.
Potential Side Reactions
While the main product of the acid-catalyzed hydration of 3-methyl-1-butene is 3-methyl-2-butanol, several side reactions can occur under certain conditions:
-
Alkene Isomerization: The acid catalyst can catalyze the isomerization of 3-methyl-1-butene to its isomers, such as 2-methyl-2-butene. This isomerization can lead to the formation of different alcohol products.
-
Dehydration: At higher temperatures, the alcohol product can undergo dehydration to regenerate the alkene. This is an equilibrium process, and the extent of dehydration depends on reaction conditions such as temperature and acid concentration.
-
Polymerization: Under certain conditions, the carbocation intermediate can undergo polymerization reactions, resulting in the formation of polymeric materials. This is more likely to occur at higher alkene concentrations and lower water concentrations.
Comparison with Other Hydration Methods
While acid-catalyzed hydration is a common method for hydrating alkenes, other methods exist, each with its advantages and disadvantages:
-
Oxymercuration-Demercuration: This method uses mercuric acetate and sodium borohydride and generally proceeds with Markovnikov regioselectivity. It offers higher regioselectivity and avoids carbocation rearrangements which are possible in acid-catalyzed hydration.
-
Hydroboration-Oxidation: This two-step process uses borane followed by oxidation with hydrogen peroxide and sodium hydroxide. It proceeds anti-Markovnikov, adding the hydroxyl group to the less substituted carbon. This method is highly regio- and stereoselective, producing alcohols with syn addition of hydrogen and hydroxide.
Conclusion
The acid-catalyzed hydration of 3-methyl-1-butene is a classic example of an electrophilic addition reaction, providing a straightforward approach to synthesizing 3-methyl-2-butanol. Understanding the reaction mechanism, regioselectivity, stereochemistry, and potential side reactions is crucial for optimizing the reaction conditions and achieving high yields. While other methods for alkene hydration exist, the acid-catalyzed approach remains a valuable tool in organic synthesis due to its simplicity and relatively accessible conditions. The choice of method depends on the desired regio- and stereochemistry of the product and the tolerance for potential side reactions. Careful control of reaction parameters, such as temperature, acid concentration, and water content, is crucial to ensure successful synthesis and maximize the yield of the desired alcohol. This detailed understanding enables effective planning and execution of the reaction, ultimately leading to efficient synthesis of desired products in organic chemistry laboratories.
Latest Posts
Latest Posts
-
Learning About Dance Dance As An Art Form And Entertainment
Apr 01, 2025
-
America The Essential Learning Edition Volume 1
Apr 01, 2025
-
Draw The Lewis Dot Diagram For A Anion
Apr 01, 2025
-
Art Labeling Activity Functions Of Antibodies
Apr 01, 2025
-
Select The Correct Iupac Name For Each Unsaturated Hydrocarbon
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Consider The Acid Catalyzed Hydration Of 3 Methyl 1 Butene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
