Complete The Subscripts On The Following Equations
Holbox
Mar 28, 2025 · 7 min read
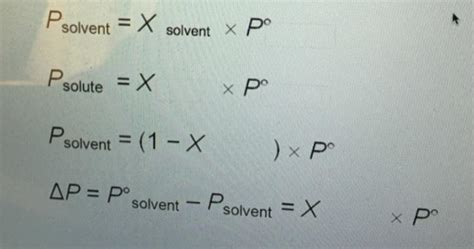
Table of Contents
- Complete The Subscripts On The Following Equations
- Table of Contents
- Completing the Subscripts in Chemical Equations: A Comprehensive Guide
- Understanding Subscripts and Coefficients
- Identifying Common Ions and Their Charges
- Writing Formulas for Ionic Compounds
- Completing Subscripts in Chemical Equations: A Step-by-Step Approach
- Common Mistakes to Avoid
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Completing the Subscripts in Chemical Equations: A Comprehensive Guide
Balancing chemical equations is a fundamental skill in chemistry. It ensures the law of conservation of mass is obeyed, meaning the number of atoms of each element remains the same on both sides of the equation. While balancing the coefficients (the numbers in front of the chemical formulas) is crucial, understanding and correctly writing subscripts is equally important. Subscripts indicate the number of atoms of a particular element within a molecule or compound. Getting these wrong fundamentally alters the chemical species involved, leading to an incorrect and potentially dangerous representation of a chemical reaction. This comprehensive guide will delve into the intricacies of completing subscripts in chemical equations, providing you with the knowledge and strategies to master this essential skill.
Understanding Subscripts and Coefficients
Before we jump into completing subscripts, let's clarify the difference between subscripts and coefficients.
-
Subscripts: These are small numbers written below and to the right of a chemical symbol. They indicate the number of atoms of that element in a single molecule or formula unit. For example, in H₂O, the subscript '2' indicates two hydrogen atoms, and the implied subscript '1' for oxygen indicates one oxygen atom. Changing a subscript changes the identity of the chemical. H₂O (water) is very different from H₂O₂ (hydrogen peroxide).
-
Coefficients: These are large numbers written in front of a chemical formula. They indicate the number of molecules or formula units of that substance involved in the reaction. For example, in the equation 2H₂ + O₂ → 2H₂O, the coefficient '2' in front of H₂ means two molecules of hydrogen gas are involved, and the coefficient '2' in front of H₂O means two molecules of water are produced. Changing a coefficient adjusts the quantity of a substance, not its identity.
Identifying Common Ions and Their Charges
Many chemical reactions involve ions—atoms or groups of atoms carrying an electric charge. Knowing common ions and their charges is crucial for correctly writing formulas and completing subscripts. Here are some examples:
-
Monatomic Ions: These are single atoms with a charge. Examples include Na⁺ (sodium ion), Cl⁻ (chloride ion), Mg²⁺ (magnesium ion), and O²⁻ (oxide ion).
-
Polyatomic Ions: These are groups of atoms with a charge. Examples include SO₄²⁻ (sulfate ion), NO₃⁻ (nitrate ion), PO₄³⁻ (phosphate ion), and NH₄⁺ (ammonium ion).
You need to memorize these common ions and their charges to correctly predict the subscripts in ionic compounds.
Writing Formulas for Ionic Compounds
Ionic compounds are formed between positively charged cations and negatively charged anions. The subscripts in the formula of an ionic compound are determined by balancing the charges to achieve electrical neutrality. The overall charge of the compound must be zero.
Example: Consider the formation of sodium chloride (NaCl). Sodium (Na) forms a +1 ion (Na⁺), and chlorine (Cl) forms a -1 ion (Cl⁻). To balance the charges, one Na⁺ ion combines with one Cl⁻ ion, resulting in the formula NaCl.
Example with different charges: Consider magnesium oxide (MgO). Magnesium (Mg) forms a +2 ion (Mg²⁺), and oxygen (O) forms a -2 ion (O²⁻). To balance the charges, one Mg²⁺ ion combines with one O²⁻ ion, resulting in the formula MgO.
Example with unequal charges: Consider aluminum oxide (Al₂O₃). Aluminum (Al) forms a +3 ion (Al³⁺), and oxygen (O) forms a -2 ion (O²⁻). To balance the charges, we need two Al³⁺ ions (total charge +6) and three O²⁻ ions (total charge -6). This gives us the formula Al₂O₃.
Method to Determine Subscripts in Ionic Compounds:
- Identify the ions and their charges.
- Use the criss-cross method: Write the magnitude of the charge of one ion as the subscript of the other ion.
- Simplify the subscripts (if necessary): If the subscripts have a common factor, divide both by that factor to obtain the simplest whole-number ratio.
Completing Subscripts in Chemical Equations: A Step-by-Step Approach
Let's illustrate the process with a few examples:
Example 1: Combustion of Methane
The unbalanced equation is: CH₄ + O₂ → CO₂ + H₂O
-
Balance the carbons: There is one carbon atom on each side, so it's already balanced.
-
Balance the hydrogens: There are four hydrogen atoms on the left (CH₄) and two on the right (H₂O). We need to add a coefficient of 2 in front of H₂O to balance the hydrogens: CH₄ + O₂ → CO₂ + 2H₂O
-
Balance the oxygens: There are two oxygen atoms on the left (O₂) and four on the right (CO₂ has two, and 2H₂O has two more). We need to add a coefficient of 2 in front of O₂: CH₄ + 2O₂ → CO₂ + 2H₂O. The equation is now balanced. Note: The subscripts remained unchanged; we only adjusted the coefficients.
Example 2: Reaction Between Iron(III) Chloride and Sodium Hydroxide
Let's assume we start with an unbalanced equation where the subscripts are not fully defined: FeCl₃ + NaOH → Fe(OH)₃ + NaCl. Here we need to balance both coefficients and ensure correct subscripts are present.
-
Verify Subscripts: The subscripts in FeCl₃, NaOH, Fe(OH)₃ and NaCl are correct based on the charges of the ions involved. Fe³⁺ combines with three Cl⁻ ions, Na⁺ combines with OH⁻, Fe³⁺ combines with three OH⁻, and Na⁺ combines with Cl⁻.
-
Balance the equation:
- Iron (Fe): One iron atom on each side – balanced.
- Chlorine (Cl): Three chlorine atoms on the left, one on the right. Add a coefficient of 3 to NaCl: FeCl₃ + NaOH → Fe(OH)₃ + 3NaCl
- Sodium (Na): One sodium atom on the left, three on the right. Add a coefficient of 3 to NaOH: FeCl₃ + 3NaOH → Fe(OH)₃ + 3NaCl
- Oxygen (O) and Hydrogen (H): Three oxygen and three hydrogen atoms on both sides – balanced.
The balanced equation is FeCl₃ + 3NaOH → Fe(OH)₃ + 3NaCl. Again, the subscripts in the formulas are unchanged; only the coefficients were adjusted to balance the equation.
Example 3: A More Complex Reaction
Consider the reaction between potassium permanganate (KMnO₄) and hydrogen peroxide (H₂O₂) in acidic solution, producing manganese(II) ions (Mn²⁺), potassium ions (K⁺), water (H₂O), and oxygen gas (O₂).
The unbalanced equation might be presented as: KMnO₄ + H₂O₂ + H⁺ → Mn²⁺ + K⁺ + H₂O + O₂
This reaction requires a more complex balancing approach using the half-reaction method, but the fundamental principle of correct subscripts remains. The subscripts in KMnO₄, H₂O₂, H₂O, and O₂ are already correct based on the known formulas of these compounds. We would then balance the equation by adjusting coefficients to ensure that atoms of each element are equal on both sides. Balancing this redox reaction is beyond the scope of this introductory guide, but it exemplifies how correct subscripts are a prerequisite for successful balancing.
Common Mistakes to Avoid
-
Confusing subscripts and coefficients: Remember, subscripts define the formula of a molecule; coefficients define the number of molecules.
-
Incorrectly writing ionic formulas: Always ensure that the charges of the ions are balanced when writing the formulas of ionic compounds. Use the criss-cross method to help you.
-
Neglecting polyatomic ions: Treat polyatomic ions as single units when balancing equations. Don't break them down into their constituent atoms.
-
Not checking your work: After balancing, always double-check that the number of atoms of each element is the same on both sides of the equation.
Conclusion
Completing subscripts correctly in chemical equations is paramount. It forms the foundation for accurate representation of chemical reactions. By understanding the concepts of ions, charges, and the rules for writing chemical formulas, you can master the skill of balancing equations and avoid common pitfalls. Remember to practice regularly, work through various examples, and always double-check your work. With consistent effort, you will develop the confidence and proficiency to confidently handle even complex chemical equations. The correct use of subscripts ensures the accurate representation and prediction of chemical behavior, making it a cornerstone of chemical understanding.
Latest Posts
Latest Posts
-
When Handling Dod Legacy Marked Material
Apr 01, 2025
-
Classify Each Phrase As A Description Of Alpha Helices
Apr 01, 2025
-
Cole Is Drafting A Legal Pleading
Apr 01, 2025
-
Discovering Music With Digital Course Materials For Discovering Music Paperback
Apr 01, 2025
-
Its Busy At The Bakery Counter
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Complete The Subscripts On The Following Equations . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
