Complete The Curved Arrow Pushing Mechanism
Holbox
Mar 28, 2025 · 6 min read
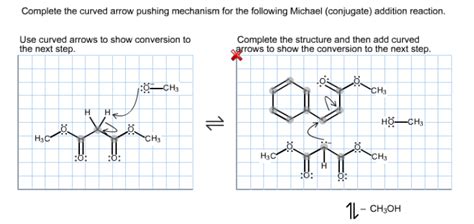
Table of Contents
- Complete The Curved Arrow Pushing Mechanism
- Table of Contents
- Mastering Curved Arrow Pushing Mechanisms: A Comprehensive Guide
- Understanding the Basics: Electron Movement and Formal Charges
- The Arrow's Tale: Origin and Destination
- Formal Charges: A Necessary Accounting
- Common Types of Curved Arrow Pushing: A Practical Overview
- 1. Proton Transfer (Acid-Base Reactions)
- 2. Nucleophilic Attack
- 3. Loss of Leaving Group
- 4. Rearrangements: Carbocation Shifts
- 5. Additions to Pi Bonds
- 6. Eliminations: Loss of a Small Molecule
- Advanced Applications and Common Mistakes
- Resonance Structures: Delocalization of Electrons
- Concerted Reactions: Simultaneous Bond Making and Breaking
- Radical Reactions: Single Electron Movement
- Common Mistakes to Avoid
- Practice Makes Perfect: Tips for Mastering Curved Arrow Pushing
- Conclusion: A Powerful Tool for Organic Chemistry
- Latest Posts
- Latest Posts
- Related Post
Mastering Curved Arrow Pushing Mechanisms: A Comprehensive Guide
Curved arrow pushing is a fundamental skill for any organic chemist. It's the visual language we use to represent the movement of electrons during a chemical reaction. Mastering this skill is crucial for understanding reaction mechanisms, predicting reaction products, and designing new synthetic routes. This comprehensive guide will walk you through the intricacies of curved arrow pushing, covering everything from basic principles to advanced applications. We'll tackle common pitfalls and provide plenty of examples to solidify your understanding.
Understanding the Basics: Electron Movement and Formal Charges
At the heart of curved arrow pushing lies the understanding of electron movement. A curved arrow always depicts the movement of two electrons. This is crucial because it dictates the changes in bonding and formal charges within the molecules involved.
The Arrow's Tale: Origin and Destination
The tail of the curved arrow originates from an electron-rich site. This could be a lone pair of electrons on an atom, a pi bond, or even a sigma bond in specific situations. The head of the arrow points to an electron-poor site, which could be a positively charged atom, a partially positive atom (δ+), or an atom capable of accepting electrons to form a new bond.
Formal Charges: A Necessary Accounting
Formal charges are essential for tracking electron redistribution during reactions. Remember, a formal charge represents the difference between the number of valence electrons an atom should have and the number it actually possesses in a molecule or ion. A correctly drawn curved arrow mechanism must accurately reflect changes in formal charges.
- Calculating Formal Charge: Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - 1/2(Bonding Electrons)
Remember to adjust formal charges after each step in your mechanism to ensure accuracy.
Common Types of Curved Arrow Pushing: A Practical Overview
Let's explore the most frequent types of electron movements you'll encounter in organic chemistry.
1. Proton Transfer (Acid-Base Reactions)
This is one of the simplest types of reactions. A curved arrow originates from a lone pair on a base (electron-rich species) and points towards the proton (H⁺) of an acid. Simultaneously, another arrow originates from the bond between the proton and the acid, pointing towards the acid's conjugate base.
Example: Reaction of ammonia (NH₃) with hydrochloric acid (HCl). The lone pair on nitrogen attacks the proton of HCl, forming a new N-H bond and leaving Cl⁻ as the conjugate base.
2. Nucleophilic Attack
Nucleophiles are electron-rich species that attack electrophilic centers (electron-deficient sites). The curved arrow originates from the nucleophile's lone pair or a pi bond and points towards the electrophilic carbon.
Example: SN2 reaction. A nucleophile attacks the electrophilic carbon from the backside, leading to inversion of configuration.
3. Loss of Leaving Group
A leaving group is an atom or group of atoms that departs with a pair of electrons. The curved arrow originates from the bond between the carbon and the leaving group, pointing towards the leaving group. This signifies the breaking of the bond and the formation of a negative charge or a neutral species on the leaving group.
Example: SN1 reactions often involve the loss of a leaving group to form a carbocation intermediate.
4. Rearrangements: Carbocation Shifts
Carbocation rearrangements involve the movement of a hydride ion (H⁻) or an alkyl group to a more stable carbocation. These rearrangements often occur to produce a more stable tertiary carbocation from a secondary or primary one. The arrow starts from the bond adjacent to the positively charged carbon and points toward the positively charged carbon. This shift involves simultaneous bond formation and bond breaking.
Example: 1,2-hydride shift in a carbocation.
5. Additions to Pi Bonds
Additions to pi bonds involve the breaking of a pi bond and the formation of two new sigma bonds. This is commonly seen in electrophilic additions to alkenes and alkynes. The arrow starts from the pi bond, and its head points towards the electrophile and the carbocation center (or other electrophilic species).
Example: Electrophilic addition of hydrogen bromide (HBr) to an alkene.
6. Eliminations: Loss of a Small Molecule
Elimination reactions involve the loss of a small molecule, such as water or hydrogen halide, from a substrate. This often leads to the formation of a pi bond. Arrows indicate bond breaking and bond formation simultaneously.
Example: Dehydration of an alcohol (E1 or E2 mechanisms).
Advanced Applications and Common Mistakes
Let's delve into some more complex aspects of curved arrow pushing and address common errors.
Resonance Structures: Delocalization of Electrons
Resonance structures depict the delocalization of electrons within a molecule or ion. They are not different molecules; instead, they represent different ways of depicting the same molecule with the same overall structure but with different distributions of electrons.
Drawing Resonance Structures: Use curved arrows to show the movement of electrons, especially lone pairs and pi electrons. Remember to maintain the overall charge and the connectivity of atoms.
Concerted Reactions: Simultaneous Bond Making and Breaking
Concerted reactions involve bond breaking and bond making happening at the same time. This requires the careful consideration of electron movements and requires using multiple arrows simultaneously. One must show the simultaneous breaking of one bond and the forming of another.
Example: SN2 reactions and Diels-Alder reactions are classic examples of concerted mechanisms.
Radical Reactions: Single Electron Movement
Unlike the previous examples that focus on two-electron movements, radical reactions involve single electron movement. The curved arrow represents the movement of a single electron, typically from a radical center to a new location. The use of a half-headed arrow (a single-barb arrow) is used to represent the movement of a single electron.
Common Mistakes to Avoid
- Incorrect Arrow Direction: Ensure the tail originates from an electron-rich center and the head points to an electron-poor center.
- Ignoring Formal Charges: Always recalculate formal charges after each arrow-pushing step.
- Violation of Octet Rule: Ensure that all atoms (except hydrogen and some exceptions like boron) have a full octet of electrons.
- Missing or Extra Arrows: Each electron movement requires a curved arrow. Don't add extra or miss necessary ones.
- Incorrect Stereochemistry: Pay attention to stereochemistry, especially in SN2 reactions and other reactions where inversion of configuration might occur.
Practice Makes Perfect: Tips for Mastering Curved Arrow Pushing
The key to mastering curved arrow pushing is practice. Work through numerous examples, starting with simple reactions and gradually progressing to more complex ones. Consult textbooks and online resources for additional practice problems. Focus on understanding the logic behind each electron movement, rather than just memorizing the arrows. Consistent practice will build your confidence and allow you to accurately predict reaction pathways.
Conclusion: A Powerful Tool for Organic Chemistry
Curved arrow pushing is not just a technical skill; it's a powerful tool that unlocks a deeper understanding of organic chemistry. By understanding electron movement, formal charges, and the various types of arrow pushing, you gain the ability to predict reaction outcomes, analyze complex mechanisms, and ultimately, design new synthetic strategies. Remember, consistent practice and attention to detail are key to mastering this fundamental skill and unlocking the vast world of organic chemistry. This comprehensive guide provides a solid foundation. Continue to practice and explore, and you'll become a confident and skilled user of curved arrow pushing.
Latest Posts
Latest Posts
-
Total Utility May Be Determined By
Apr 01, 2025
-
Strategic Intent Refers To A Situation Where A Company
Apr 01, 2025
-
Which Of These Events Occurred First
Apr 01, 2025
-
What Are Homemade Dividends And Why Would Investors Make Them
Apr 01, 2025
-
Which Of The Following Is A Normative Statement
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Complete The Curved Arrow Pushing Mechanism . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
