Classify These Molecules As Polar Or Nonpolar.
Holbox
Mar 27, 2025 · 6 min read
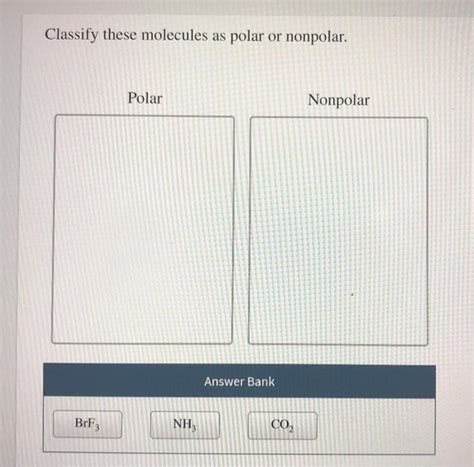
Table of Contents
- Classify These Molecules As Polar Or Nonpolar.
- Table of Contents
- Classify These Molecules as Polar or Nonpolar: A Comprehensive Guide
- Understanding Polarity: The Basics
- Electronegativity: The Electron Tug-of-War
- Bond Polarity: The Foundation of Molecular Polarity
- Classifying Molecules: A Step-by-Step Approach
- Step 1: Identify the Bonds
- Step 2: Analyze Molecular Geometry
- Step 3: Determine Overall Polarity
- Examples: Classifying Molecules
- 1. Carbon Dioxide (CO₂)
- 2. Water (H₂O)
- 3. Methane (CH₄)
- 4. Ammonia (NH₃)
- 5. Carbon Tetrachloride (CCl₄)
- 6. Sulfur Dioxide (SO₂)
- Advanced Considerations: Factors Influencing Polarity
- Conclusion: Mastering Molecular Polarity
- Latest Posts
- Related Post
Classify These Molecules as Polar or Nonpolar: A Comprehensive Guide
Determining whether a molecule is polar or nonpolar is a fundamental concept in chemistry, crucial for understanding its properties and behavior. This comprehensive guide will delve into the intricacies of molecular polarity, providing you with the knowledge and tools to classify molecules accurately. We'll explore the concepts of electronegativity, bond polarity, molecular geometry, and how these factors interplay to dictate a molecule's overall polarity.
Understanding Polarity: The Basics
The polarity of a molecule hinges on the distribution of electron density. If the electrons are shared equally between atoms, the molecule is nonpolar. However, if the electrons are pulled more towards one atom, creating a dipole moment, the molecule is polar. This unequal sharing stems from differences in electronegativity.
Electronegativity: The Electron Tug-of-War
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Elements on the right side of the periodic table (excluding noble gases) are highly electronegative, while those on the left are less electronegative. The larger the difference in electronegativity between two atoms, the more polar the bond between them.
Bond Polarity: The Foundation of Molecular Polarity
A polar bond forms when there's a significant difference in electronegativity between the bonded atoms. This difference creates a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. A nonpolar bond occurs when the electronegativity difference is negligible, resulting in an even distribution of electrons.
Classifying Molecules: A Step-by-Step Approach
Classifying a molecule as polar or nonpolar requires a systematic approach, considering both bond polarity and molecular geometry.
Step 1: Identify the Bonds
First, examine each bond within the molecule. Determine the electronegativity difference between the bonded atoms. You can use a Pauling electronegativity chart as a reference. If the difference is significant (generally greater than 0.4), the bond is considered polar. If the difference is small (less than 0.4), the bond is considered nonpolar.
Step 2: Analyze Molecular Geometry
Molecular geometry, or the three-dimensional arrangement of atoms, plays a crucial role in determining overall molecular polarity. Even if a molecule contains polar bonds, the molecule itself might be nonpolar if the geometry cancels out the individual bond dipoles.
Symmetrical molecules: In molecules with symmetrical shapes like linear (CO<sub>2</sub>), trigonal planar (BF<sub>3</sub>), and tetrahedral (CCl<sub>4</sub>), the individual bond dipoles often cancel each other out, resulting in a nonpolar molecule. The vectors representing the bond dipoles sum to zero.
Asymmetrical molecules: In molecules with asymmetrical shapes like bent (H<sub>2</sub>O), trigonal pyramidal (NH<sub>3</sub>), and T-shaped molecules, the individual bond dipoles do not cancel each other out, leading to a net dipole moment and thus, a polar molecule. The vectors representing the bond dipoles do not sum to zero.
Step 3: Determine Overall Polarity
Once you've analyzed the bond polarities and molecular geometry, combine this information to determine the overall polarity. If the molecule has polar bonds and an asymmetrical geometry, it will be polar. If the molecule has nonpolar bonds, or if it has polar bonds but a symmetrical geometry that cancels out the bond dipoles, it will be nonpolar.
Examples: Classifying Molecules
Let's classify some molecules using the steps outlined above:
1. Carbon Dioxide (CO₂)
- Step 1: Bond Polarity: Oxygen is more electronegative than carbon, so each C=O bond is polar.
- Step 2: Molecular Geometry: CO<sub>2</sub> has a linear geometry.
- Step 3: Overall Polarity: The two polar C=O bonds are oriented in opposite directions, and their dipole moments cancel each other out. Therefore, CO₂ is a nonpolar molecule.
2. Water (H₂O)
- Step 1: Bond Polarity: Oxygen is more electronegative than hydrogen, so each O-H bond is polar.
- Step 2: Molecular Geometry: H<sub>2</sub>O has a bent geometry.
- Step 3: Overall Polarity: The two polar O-H bonds do not cancel each other out due to the bent geometry. Therefore, H₂O is a polar molecule.
3. Methane (CH₄)
- Step 1: Bond Polarity: Carbon and hydrogen have similar electronegativities, resulting in slightly polar C-H bonds (the difference is small enough to often be considered nonpolar).
- Step 2: Molecular Geometry: CH<sub>4</sub> has a tetrahedral geometry.
- Step 3: Overall Polarity: Even with slightly polar bonds, the tetrahedral symmetry causes the bond dipoles to cancel each other out. Therefore, CH₄ is considered a nonpolar molecule.
4. Ammonia (NH₃)
- Step 1: Bond Polarity: Nitrogen is more electronegative than hydrogen, so each N-H bond is polar.
- Step 2: Molecular Geometry: NH<sub>3</sub> has a trigonal pyramidal geometry.
- Step 3: Overall Polarity: The three polar N-H bonds and the lone pair on nitrogen create an asymmetrical distribution of electron density. Therefore, NH₃ is a polar molecule.
5. Carbon Tetrachloride (CCl₄)
- Step 1: Bond Polarity: Chlorine is more electronegative than carbon, resulting in polar C-Cl bonds.
- Step 2: Molecular Geometry: CCl<sub>4</sub> has a tetrahedral geometry.
- Step 3: Overall Polarity: The four polar C-Cl bonds are symmetrically arranged, and their dipole moments cancel each other out. Therefore, CCl₄ is a nonpolar molecule.
6. Sulfur Dioxide (SO₂)
- Step 1: Bond Polarity: Oxygen is more electronegative than sulfur, creating polar S=O bonds.
- Step 2: Molecular Geometry: SO₂ has a bent geometry.
- Step 3: Overall Polarity: The bent geometry prevents the dipole moments from canceling each other out. Therefore, SO₂ is a polar molecule.
Advanced Considerations: Factors Influencing Polarity
While electronegativity and molecular geometry are the primary determinants of polarity, other factors can influence it:
- Resonance: In molecules with resonance structures, the delocalization of electrons can affect the overall dipole moment.
- Lone Pairs: Lone pairs of electrons contribute significantly to a molecule's polarity, as they create regions of higher electron density.
- Inductive Effects: The presence of electronegative or electropositive substituents can induce a dipole moment in nearby bonds.
Conclusion: Mastering Molecular Polarity
Understanding molecular polarity is essential for predicting the physical and chemical properties of molecules. By systematically analyzing bond polarity and molecular geometry, you can accurately classify molecules as polar or nonpolar. This knowledge is fundamental to various fields, including biochemistry, materials science, and pharmaceutical chemistry. Remember to always consider electronegativity differences, molecular geometry, and any other influencing factors to make a comprehensive and accurate determination. With practice and a clear understanding of the concepts outlined in this guide, you can confidently classify molecules based on their polarity.
Latest Posts
Related Post
Thank you for visiting our website which covers about Classify These Molecules As Polar Or Nonpolar. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.