Choose The Correct Name For The Following Compound
Holbox
Mar 28, 2025 · 7 min read
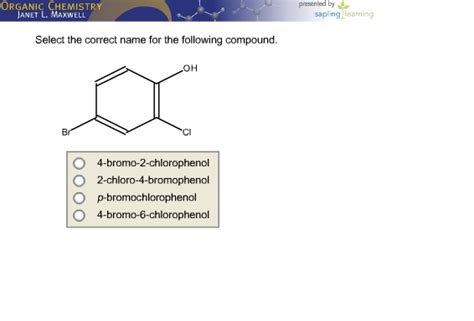
Table of Contents
- Choose The Correct Name For The Following Compound
- Table of Contents
- Choosing the Correct Name for a Chemical Compound: A Comprehensive Guide
- Understanding IUPAC Nomenclature
- Key Considerations in Compound Naming
- Naming Inorganic Compounds
- Naming Organic Compounds
- Dealing with Complex Structures
- Common Pitfalls and How to Avoid Them
- Practical Exercises
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Choosing the Correct Name for a Chemical Compound: A Comprehensive Guide
Naming chemical compounds might seem like a straightforward task, but it's a nuanced process governed by a set of strict rules established by the International Union of Pure and Applied Chemistry (IUPAC). This guide dives deep into the intricacies of chemical nomenclature, providing a comprehensive framework for accurately and consistently naming various types of compounds. We'll explore different naming conventions, common pitfalls, and practical examples to help you master this essential skill.
Understanding IUPAC Nomenclature
The IUPAC nomenclature is the internationally accepted standard for naming chemical compounds. Its purpose is to ensure unambiguous communication between scientists worldwide. The system is hierarchical, with different rules applying to different classes of compounds. The fundamental principle is to create a unique name that reflects the compound's structure and composition. This ensures that everyone, regardless of their background or language, can understand precisely which chemical substance is being discussed.
Key Considerations in Compound Naming
Before delving into specific naming conventions, let's outline some crucial factors to keep in mind:
-
Type of Compound: The first step is identifying the type of compound you're dealing with. Is it an inorganic compound (typically involving elements other than carbon) or an organic compound (containing carbon and hydrogen)? Different rules apply to each category.
-
Functional Groups: Organic compounds are categorized based on their functional groups – specific atoms or groups of atoms that determine the compound's chemical properties and reactivity. Identifying the principal functional group is crucial in assigning the correct name.
-
Parent Chain/Structure: For organic compounds, the parent chain (the longest continuous carbon chain) or the parent structure (the main cyclic structure) forms the basis of the name. Substituents (atoms or groups attached to the parent chain/structure) are named accordingly.
-
Numbering and Alphabetical Order: The parent chain/structure is numbered to indicate the position of substituents. Substituent names are listed alphabetically, regardless of their position on the chain/structure.
Naming Inorganic Compounds
Inorganic compounds comprise a vast range of substances, and their naming follows specific conventions depending on the type of compound:
1. Binary Ionic Compounds (Metal and Non-metal):
These compounds consist of a metal cation and a non-metal anion. The name of the metal is written first, followed by the name of the non-metal with its ending changed to "-ide." If the metal has multiple oxidation states (e.g., iron, copper), the oxidation state is indicated using Roman numerals in parentheses.
- Example: FeCl₃ is named Iron(III) chloride, indicating iron's +3 oxidation state. FeCl₂ is Iron(II) chloride.
2. Binary Covalent Compounds (Non-metal and Non-metal):
These compounds are named using prefixes to indicate the number of atoms of each element. The prefix "mono-" is typically omitted for the first element unless it is needed for clarity. The second element's name ends in "-ide."
- Example: CO₂ is named Carbon dioxide, while N₂O₄ is Dinitrogen tetroxide.
3. Acids:
Acids are compounds that release hydrogen ions (H⁺) in aqueous solution. Their names depend on the anion they form.
- Example: HCl (hydrochloric acid), HNO₃ (nitric acid), H₂SO₄ (sulfuric acid). Note the use of "hydro-" for binary acids and the "-ic" acid ending for oxoacids.
4. Salts:
Salts are ionic compounds formed from the reaction of an acid and a base. Their names are derived from the cation and anion.
- Example: NaCl (sodium chloride), K₂SO₄ (potassium sulfate).
Naming Organic Compounds
Organic compounds are significantly more diverse than inorganic compounds, and their nomenclature is more intricate. We'll focus on some key classes:
1. Alkanes:
Alkanes are hydrocarbons containing only single carbon-carbon bonds. Their names are based on the number of carbon atoms: methane (CH₄), ethane (C₂H₆), propane (C₃H₈), butane (C₄H₁₀), and so on. Longer chains follow a systematic naming convention.
2. Alkenes and Alkynes:
Alkenes contain at least one carbon-carbon double bond, and alkynes contain at least one carbon-carbon triple bond. The names are based on the corresponding alkane, with the suffix "-ene" for alkenes and "-yne" for alkynes. The position of the multiple bond is indicated by a number.
- Example: CH₂=CHCH₃ is propene (the double bond is between carbons 1 and 2). CH≡CH is ethyne.
3. Alcohols:
Alcohols contain a hydroxyl group (-OH) attached to a carbon atom. Their names are derived from the corresponding alkane, with the suffix "-ol." The position of the hydroxyl group is indicated by a number.
- Example: CH₃CH₂OH is ethanol. CH₃CH(OH)CH₃ is propan-2-ol.
4. Ketones:
Ketones contain a carbonyl group (=O) bonded to two carbon atoms. Their names are based on the corresponding alkane, with the suffix "-one." The position of the carbonyl group is indicated by a number.
- Example: CH₃COCH₃ is propanone (or acetone).
5. Aldehydes:
Aldehydes contain a carbonyl group (=O) bonded to one carbon atom and one hydrogen atom. Their names are based on the corresponding alkane, with the suffix "-al."
- Example: CH₃CHO is ethanal.
6. Carboxylic Acids:
Carboxylic acids contain a carboxyl group (-COOH). Their names are based on the corresponding alkane, with the suffix "-oic acid."
- Example: CH₃COOH is ethanoic acid (or acetic acid).
7. Amines:
Amines contain a nitrogen atom bonded to one or more carbon atoms. Their names are derived from the corresponding alkane, with the suffix "-amine." The position of the amino group is indicated by a number.
- Example: CH₃NH₂ is methanamine.
8. Ethers:
Ethers contain an oxygen atom bonded to two carbon atoms. They are named by listing the alkyl groups attached to the oxygen atom, followed by the word "ether."
- Example: CH₃OCH₃ is dimethyl ether.
9. Esters:
Esters are derived from carboxylic acids and alcohols. They are named by first naming the alkyl group from the alcohol, followed by the name of the carboxylate ion derived from the carboxylic acid.
- Example: CH₃COOCH₂CH₃ is ethyl ethanoate.
10. Substituted Compounds:
Many organic compounds contain substituents – atoms or groups attached to the parent chain or structure. These substituents are named as prefixes, and their positions are indicated by numbers. The prefixes are listed alphabetically.
- Example: 2-methylpropane.
Dealing with Complex Structures
For complex molecules with multiple functional groups or substituents, determining the principal functional group and following the IUPAC rules meticulously is crucial. Prioritizing the principal functional group and assigning the correct numbering system are essential steps in accurate naming. Referencing comprehensive chemical nomenclature guides and using chemical drawing software can significantly aid in tackling complex structures.
Common Pitfalls and How to Avoid Them
Several common errors can occur when naming chemical compounds:
-
Incorrect numbering: Ensure the parent chain is numbered to give the substituents the lowest possible numbers.
-
Ignoring alphabetical order: Substituents must be listed alphabetically.
-
Incorrect use of prefixes: Pay close attention to the prefixes used to indicate the number of atoms or groups.
-
Misidentification of functional groups: Carefully identify the principal functional group to determine the correct suffix.
-
Overlooking stereochemistry: For compounds exhibiting stereoisomerism (e.g., cis-trans isomerism, enantiomers), the stereochemical descriptors should be included in the name.
Practical Exercises
To solidify your understanding, practice naming various compounds. Start with simpler structures and gradually progress to more complex ones. Online resources and textbooks offer numerous exercises and examples to aid in your practice. Remember, consistency and attention to detail are key to mastering chemical nomenclature.
Conclusion
Accurately naming chemical compounds is a cornerstone of effective communication in chemistry. By understanding the fundamental principles of IUPAC nomenclature and following the guidelines meticulously, you can confidently and correctly name a wide array of compounds. Regular practice and a keen eye for detail will significantly improve your skills in this essential area of chemistry. Remember to always consult reputable resources and seek clarification when in doubt. The accuracy of chemical nomenclature is critical for scientific accuracy and safety.
Latest Posts
Latest Posts
-
To Avoid Fatigue When Should Team Roles Alternate Providing Compressions
Apr 01, 2025
-
When Handling Dod Legacy Marked Material
Apr 01, 2025
-
Classify Each Phrase As A Description Of Alpha Helices
Apr 01, 2025
-
Cole Is Drafting A Legal Pleading
Apr 01, 2025
-
Discovering Music With Digital Course Materials For Discovering Music Paperback
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Choose The Correct Name For The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
