Choose The Best Reagents To Complete The Reaction Shown Below
Holbox
Mar 14, 2025 · 6 min read
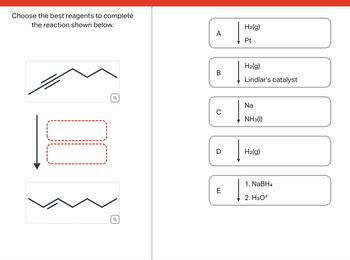
Table of Contents
- Choose The Best Reagents To Complete The Reaction Shown Below
- Table of Contents
- Choosing the Best Reagents for Organic Reactions: A Comprehensive Guide
- Understanding the Reaction: A Foundation for Reagent Selection
- 1. Identifying the Functional Groups and their Reactivity
- 2. Defining the Desired Product and its Stereochemistry
- 3. Analyzing the Reaction Mechanism
- Key Factors Influencing Reagent Selection
- 1. Chemoselectivity: Targeting Specific Functional Groups
- 2. Regioselectivity: Controlling the Reaction Site
- 3. Stereoselectivity: Controlling the Product's Stereochemistry
- 4. Reaction Conditions: Temperature, Solvent, and Atmosphere
- 5. Cost and Availability of Reagents
- 6. Toxicity and Environmental Impact
- Examples of Reagent Selection in Different Reactions
- 1. Oxidation of Alcohols:
- 2. Reduction of Carbonyl Compounds:
- 3. Alkylation of Carbonyl Compounds:
- 4. Esterification:
- Conclusion: A Strategic Approach to Reagent Selection
- Latest Posts
- Related Post
Choosing the Best Reagents for Organic Reactions: A Comprehensive Guide
Selecting the appropriate reagents is paramount in organic synthesis. The success of a reaction, including yield, selectivity, and overall efficiency, hinges on this crucial decision. This comprehensive guide delves into the strategic considerations involved in choosing the best reagents to complete a given reaction, using various examples and illustrating the importance of understanding reaction mechanisms and reagent properties. We'll explore factors like chemoselectivity, regioselectivity, stereoselectivity, and reaction conditions, highlighting why certain reagents are superior choices over others for specific transformations.
Understanding the Reaction: A Foundation for Reagent Selection
Before even considering specific reagents, a thorough understanding of the desired transformation is essential. This involves:
1. Identifying the Functional Groups and their Reactivity
The presence and nature of functional groups dictate the possible reactions and the reagents that can be effectively used. For example, alcohols can undergo various transformations like oxidation, esterification, or conversion to halides, each requiring a different reagent. Recognizing the inherent reactivity of each functional group is crucial. A carbonyl group, for instance, can undergo nucleophilic addition, reduction, or condensation reactions, all influenced by the choice of reagent.
2. Defining the Desired Product and its Stereochemistry
The desired product, including its stereochemistry (if relevant), guides the selection of reagents. For instance, if a specific enantiomer or diastereomer is required, stereoselective reagents must be employed. Understanding the stereochemical outcome of a reaction is crucial to predicting the success of a synthesis.
3. Analyzing the Reaction Mechanism
A deep understanding of the reaction mechanism allows for a more informed selection of reagents. Knowing the steps involved, the intermediates formed, and the rate-determining step can help predict the efficiency and selectivity of the reaction. This mechanistic insight allows the chemist to choose reagents that can favorably influence each step, potentially avoiding side reactions or improving yields.
Key Factors Influencing Reagent Selection
Several critical factors influence the selection of optimal reagents for a specific organic transformation:
1. Chemoselectivity: Targeting Specific Functional Groups
Chemoselectivity refers to the ability of a reagent to react preferentially with one functional group in the presence of other, potentially reactive, groups. This is particularly important in complex molecules containing multiple functional groups. For example, in a molecule containing both an alcohol and an alkene, a reagent that selectively reacts with the alcohol while leaving the alkene untouched is required. Protecting groups are often employed to achieve chemoselectivity.
Example: Selective reduction of a ketone in the presence of an ester requires a carefully chosen reducing agent, such as sodium borohydride (NaBH4), which selectively reduces ketones over esters.
2. Regioselectivity: Controlling the Reaction Site
Regioselectivity concerns the preferential reaction at one particular site within a molecule. This is especially important in reactions involving multiple possible reaction sites, such as electrophilic aromatic substitution or addition reactions to unsaturated systems. Regioselectivity often depends on the directing effects of substituents already present in the molecule and the nature of the reagent.
Example: Electrophilic aromatic substitution reactions are highly regiospecific, influenced by the directing effects of substituents (ortho/para or meta directing). The choice of electrophile and the nature of the substituent dictates the regioselectivity.
3. Stereoselectivity: Controlling the Product's Stereochemistry
Stereoselectivity refers to the preferential formation of one stereoisomer (enantiomer or diastereomer) over others. This is critical when obtaining a specific stereoisomer is necessary, particularly in pharmaceutical synthesis. Chiral reagents or catalysts are frequently employed to achieve stereoselective transformations.
Example: Sharpless epoxidation is a highly stereoselective reaction that uses a chiral catalyst to produce enantiomerically pure epoxides from allylic alcohols.
4. Reaction Conditions: Temperature, Solvent, and Atmosphere
Reaction conditions profoundly influence the outcome of the reaction. The temperature, solvent, and atmosphere (e.g., inert atmosphere to prevent oxidation) can impact the reaction rate, selectivity, and yield. Optimization of these conditions is crucial for maximizing the desired product formation.
Example: Grignard reactions, highly sensitive to moisture and oxygen, require anhydrous conditions and inert atmosphere.
5. Cost and Availability of Reagents
While efficacy is paramount, practical considerations like cost and availability of reagents are essential. Using expensive or scarce reagents might not be feasible for large-scale synthesis. A balance between reactivity and practicality is often required.
6. Toxicity and Environmental Impact
The toxicity of reagents and the environmental impact of their use are increasingly important considerations. Greener chemistry principles emphasize the use of less hazardous reagents and solvents, reducing waste and minimizing environmental harm.
Examples of Reagent Selection in Different Reactions
Let's examine some specific examples to illustrate the principles discussed:
1. Oxidation of Alcohols:
- Primary alcohols to aldehydes: Pyridinium chlorochromate (PCC) is often preferred for its ability to oxidize primary alcohols selectively to aldehydes without further oxidation to carboxylic acids. Other reagents like Swern oxidation provide alternatives, offering different selectivities and reaction conditions.
- Primary alcohols to carboxylic acids: Jones oxidation (chromic acid) is a powerful reagent for converting primary alcohols to carboxylic acids. However, its strong oxidizing power might lead to over-oxidation of other functional groups. Alternative milder options include Dess-Martin periodinane.
- Secondary alcohols to ketones: Jones oxidation, PCC, or even milder methods like DMP (Dess-Martin periodinane) are suitable for oxidizing secondary alcohols to ketones.
2. Reduction of Carbonyl Compounds:
- Ketones and aldehydes to alcohols: Sodium borohydride (NaBH4) is a mild reducing agent commonly used for reducing ketones and aldehydes to their corresponding alcohols. Lithium aluminum hydride (LiAlH4) is a stronger reducing agent that can also reduce esters, carboxylic acids, and amides. The choice depends on the presence of other reducible functional groups.
- Esters to alcohols: LiAlH4 is a powerful reagent for reducing esters to primary alcohols. NaBH4 is generally ineffective for this transformation.
- Nitriles to amines: Lithium aluminum hydride (LiAlH4) is also effective in reducing nitriles to primary amines.
3. Alkylation of Carbonyl Compounds:
- Grignard reagents: Organomagnesium halides (Grignard reagents) are powerful nucleophiles used to alkylate carbonyl compounds. They react with aldehydes and ketones to form secondary and tertiary alcohols, respectively.
- Organolithium reagents: Organolithium reagents are even stronger nucleophiles than Grignard reagents and are used in similar alkylation reactions.
4. Esterification:
- Fischer esterification: This classic reaction uses a carboxylic acid and an alcohol in the presence of an acid catalyst (like sulfuric acid) to form an ester. The choice of acid catalyst can influence reaction rate and selectivity.
Conclusion: A Strategic Approach to Reagent Selection
Choosing the best reagents for an organic reaction is a multifaceted process that requires a deep understanding of reaction mechanisms, functional group reactivity, and selectivity considerations. By carefully considering chemoselectivity, regioselectivity, stereoselectivity, reaction conditions, cost, toxicity, and environmental impact, chemists can select the most appropriate reagents to achieve high yields, excellent selectivity, and efficient syntheses. This comprehensive approach leads to successful and sustainable organic transformations. Continuous learning and exploration of new reagents and reaction conditions are crucial for advancing the field of organic chemistry and developing more efficient and environmentally friendly synthetic methods. Remember to always consult reputable organic chemistry textbooks and resources to expand your knowledge and refine your reagent selection skills.
Latest Posts
Related Post
Thank you for visiting our website which covers about Choose The Best Reagents To Complete The Reaction Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.