Carbohydrates Have A Blank______ Ratio Of Hydrogen To Oxygen.
Holbox
May 12, 2025 · 5 min read
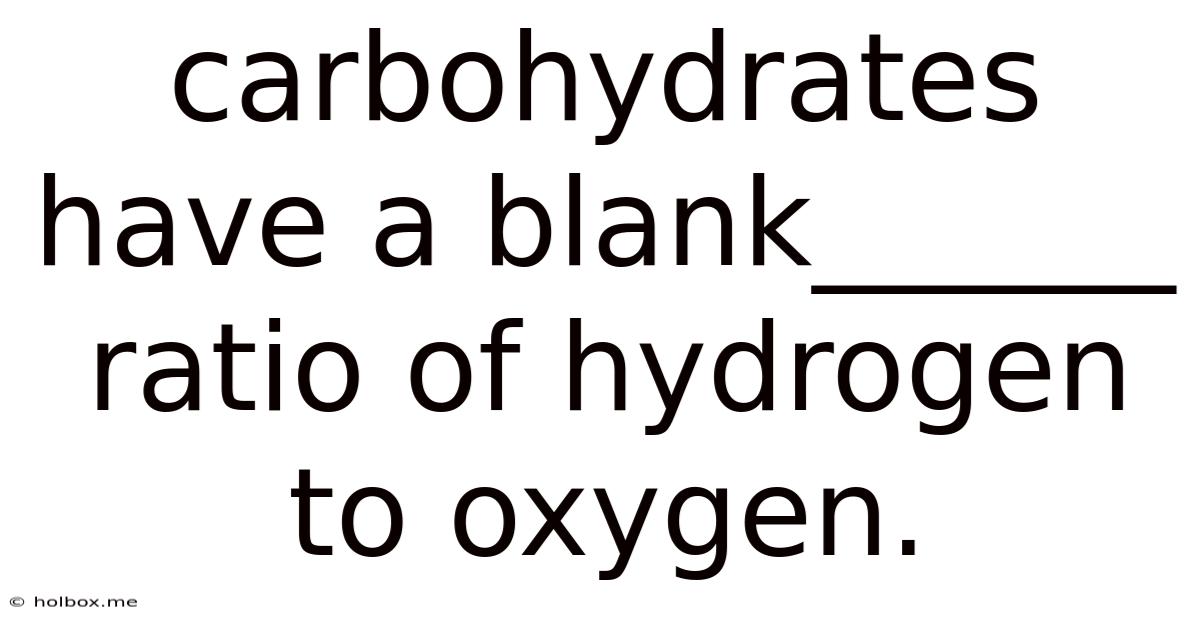
Table of Contents
- Carbohydrates Have A Blank______ Ratio Of Hydrogen To Oxygen.
- Table of Contents
- Carbohydrates: The 2:1 Hydrogen-to-Oxygen Ratio
- Understanding the Chemical Formula of Carbohydrates
- The Significance of the 2:1 Ratio
- Different Types of Carbohydrates and their 2:1 Ratio
- 1. Monosaccharides: The Simplest Sugars
- 2. Disaccharides: Double Sugars
- 3. Oligosaccharides: Short Chains of Sugars
- 4. Polysaccharides: Complex Carbohydrates
- Exceptions and Deviations from the 2:1 Ratio
- The Importance of Carbohydrates in the Body
- Carbohydrates as an Energy Source
- Carbohydrates in Other Biological Functions
- Conclusion: The 2:1 Ratio – A Defining Feature
- Latest Posts
- Related Post
Carbohydrates: The 2:1 Hydrogen-to-Oxygen Ratio
Carbohydrates are essential macronutrients, forming a cornerstone of our diet and playing crucial roles in various biological processes. Understanding their fundamental chemical structure is key to grasping their function and significance in human health and metabolism. One of the defining characteristics of carbohydrates is their 2:1 hydrogen-to-oxygen ratio. This consistent ratio is a defining feature and reflects the core chemical composition of carbohydrates. Let's delve deeper into this fascinating aspect of carbohydrate chemistry.
Understanding the Chemical Formula of Carbohydrates
The basic building blocks of carbohydrates are simple sugars, also known as monosaccharides. These monosaccharides typically follow a general chemical formula of (CH₂O)ₙ, where 'n' represents the number of carbon atoms. Glucose, the most common monosaccharide, has the formula C₆H₁₂O₆. Notice the ratio of hydrogen to oxygen atoms? It's 2:1 (12:6 simplified). This 2:1 ratio is a hallmark of carbohydrates and is directly related to their structure and how they are metabolized by the body.
The Significance of the 2:1 Ratio
This consistent ratio isn't just a coincidence. It reflects the fact that carbohydrates are essentially hydrated carbons – essentially carbon atoms with water molecules attached. The hydrogen atoms are bonded to the carbon atoms, while the oxygen atoms are present as hydroxyl (-OH) groups. This structure allows for the formation of various complex carbohydrate molecules through the process of dehydration synthesis, where water molecules are removed to link monosaccharides together.
Different Types of Carbohydrates and their 2:1 Ratio
The 2:1 hydrogen-to-oxygen ratio applies across the entire spectrum of carbohydrates, regardless of their complexity. Let's examine the different types:
1. Monosaccharides: The Simplest Sugars
Monosaccharides, such as glucose, fructose, and galactose, are the simplest form of carbohydrates. They are single sugar units and directly exhibit the 2:1 hydrogen-to-oxygen ratio in their chemical formula. For example:
- Glucose (C₆H₁₂O₆): A primary source of energy for cells.
- Fructose (C₆H₁₂O₆): Found in fruits and honey.
- Galactose (C₆H₁₂O₆): A component of lactose (milk sugar).
All three have the same ratio, demonstrating the consistency of this characteristic.
2. Disaccharides: Double Sugars
Disaccharides are formed by the joining of two monosaccharides through a glycosidic bond. This bond formation involves the removal of a water molecule (dehydration synthesis), but the overall hydrogen-to-oxygen ratio remains 2:1. Examples include:
- Sucrose (C₁₂H₂₂O₁₁): Table sugar, formed from glucose and fructose.
- Lactose (C₁₂H₂₂O₁₁): Milk sugar, formed from glucose and galactose.
- Maltose (C₁₂H₂₂O₁₁): Malt sugar, formed from two glucose molecules.
Even though a water molecule is removed during the formation of a disaccharide, the resultant molecule still maintains the characteristic 2:1 ratio.
3. Oligosaccharides: Short Chains of Sugars
Oligosaccharides contain a small number of monosaccharides linked together (typically 3 to 10). Similar to disaccharides, the formation of glycosidic bonds involves the removal of water molecules, but the overall hydrogen-to-oxygen ratio is preserved.
4. Polysaccharides: Complex Carbohydrates
Polysaccharides are long chains of monosaccharides linked together, forming complex carbohydrate structures. Examples include:
- Starch: A storage polysaccharide in plants, composed of amylose and amylopectin, both of which are built from glucose units.
- Glycogen: The storage polysaccharide in animals, also composed of glucose units.
- Cellulose: A structural polysaccharide in plants, providing rigidity to cell walls. It's also composed of glucose units, but with a different linkage compared to starch and glycogen.
- Chitin: A structural polysaccharide found in the exoskeletons of insects and crustaceans.
While the individual monosaccharide units maintain their 2:1 ratio, the overall ratio in polysaccharides can slightly deviate due to the loss of water molecules during polymerization and the presence of branching points in some polysaccharides (e.g., amylopectin and glycogen). However, the deviation is minimal and doesn't negate the fundamental 2:1 characteristic.
Exceptions and Deviations from the 2:1 Ratio
While the 2:1 hydrogen-to-oxygen ratio is a defining characteristic of carbohydrates, there are a few exceptions and nuances to consider. Some modified carbohydrates might have slightly different ratios due to the addition of other functional groups. For example, some derivatives of monosaccharides may involve the addition of phosphate groups, affecting the hydrogen-to-oxygen ratio. However, these are relatively rare exceptions and do not invalidate the general rule.
The Importance of Carbohydrates in the Body
The consistent 2:1 hydrogen-to-oxygen ratio in carbohydrates is directly linked to their crucial metabolic roles. The structure allows for efficient energy storage and release. The body breaks down carbohydrates through a series of metabolic pathways, ultimately releasing energy in the form of ATP (adenosine triphosphate). The readily available hydrogen atoms within the carbohydrate structure are essential in this energy production process.
Carbohydrates as an Energy Source
Carbohydrates are the primary source of energy for the body. Glucose, derived from the breakdown of carbohydrates, is used by cells to produce ATP through cellular respiration. The efficient energy storage and release from glucose is a direct consequence of its chemical structure and 2:1 hydrogen-to-oxygen ratio.
Carbohydrates in Other Biological Functions
Beyond energy provision, carbohydrates also play crucial roles in:
- Structural support: Cellulose provides structural support in plant cell walls.
- Cell signaling: Carbohydrates are involved in cell-cell communication and recognition.
- Glycosylation: Carbohydrates are attached to proteins and lipids, modifying their function and properties.
Conclusion: The 2:1 Ratio – A Defining Feature
The consistent 2:1 hydrogen-to-oxygen ratio is a defining characteristic of carbohydrates, reflecting their fundamental chemical structure and influencing their various biological functions. While minor exceptions exist, this ratio remains a crucial identifier and explains much about the importance of carbohydrates in biological systems and their role as a primary energy source. Understanding this ratio provides a solid foundation for comprehending the chemistry and biological significance of these essential macromolecules. From the simplest monosaccharides to the complex polysaccharides, this ratio serves as a unifying theme, highlighting the remarkable efficiency and elegance of carbohydrate structure and function. This knowledge is critical for understanding nutritional guidelines, metabolic processes, and the overall impact of carbohydrates on human health and well-being. Further research into carbohydrate metabolism continues to reveal more details about the intricacies of these vital molecules and their role in maintaining life processes.
Latest Posts
Related Post
Thank you for visiting our website which covers about Carbohydrates Have A Blank______ Ratio Of Hydrogen To Oxygen. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.