Can Tyrosine Form Hydrogen Bonds With Its Side Chain
Holbox
Mar 13, 2025 · 6 min read
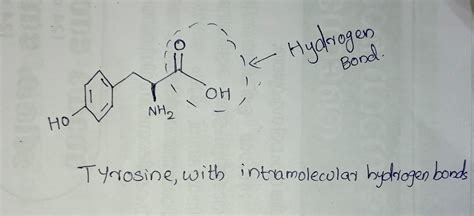
Table of Contents
Can Tyrosine Form Hydrogen Bonds with its Side Chain? Exploring the intricacies of Tyrosine's Hydrogen Bonding Capabilities
Tyrosine, a crucial aromatic amino acid, plays a vital role in various biological processes. Its unique structure, characterized by a phenolic hydroxyl group on its side chain, raises an intriguing question: can tyrosine form hydrogen bonds with its own side chain? The answer, while seemingly straightforward, delves into the complexities of molecular interactions and conformational flexibility. This article will explore this question in detail, examining the structural features of tyrosine, the nature of hydrogen bonds, and the factors influencing intramolecular hydrogen bonding in tyrosine.
Understanding Tyrosine's Structure
Tyrosine (Tyr, Y) is classified as a nonpolar aromatic amino acid. Its side chain comprises a benzene ring with a hydroxyl (-OH) group attached. This hydroxyl group is the key player in hydrogen bonding, acting as both a hydrogen bond donor and acceptor. The benzene ring itself is relatively hydrophobic, but the polar hydroxyl group introduces significant amphipathic character to the side chain. This duality influences tyrosine's interactions within proteins and its overall functionality.
The Fundamentals of Hydrogen Bonding
Hydrogen bonds are a special type of dipole-dipole attraction between molecules, not a true chemical bond. They occur when a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen or nitrogen) is attracted to another electronegative atom in a different molecule or part of the same molecule. In the case of tyrosine, the hydrogen atom of the hydroxyl group can form a hydrogen bond with the oxygen atom of the same hydroxyl group, resulting in an intramolecular hydrogen bond.
Can Tyrosine's Side Chain Form Intramolecular Hydrogen Bonds?
The answer is yes, but with caveats. The ability of tyrosine's side chain to form intramolecular hydrogen bonds hinges on several critical factors:
1. Conformational Flexibility: The side chain of tyrosine possesses rotational freedom around its bonds, allowing it to adopt various conformations. Only specific conformations allow the hydroxyl hydrogen to be in close proximity to the hydroxyl oxygen, enabling hydrogen bond formation. These conformations are often influenced by the local environment within a protein's three-dimensional structure.
2. Steric Hindrance: The bulky benzene ring attached to the hydroxyl group can hinder the formation of an intramolecular hydrogen bond. The spatial arrangement needs to be favorable to avoid steric clashes that would prevent the hydrogen bond from forming. This constraint further limits the conformations capable of supporting intramolecular hydrogen bonding.
3. Solvent Effects: The surrounding solvent molecules also play a critical role. In aqueous solutions, water molecules compete with the hydroxyl group for hydrogen bonding. If the hydrogen bonds formed with water are stronger, they can effectively prevent the formation of an intramolecular hydrogen bond within the tyrosine side chain.
4. Proximity to other functional groups: The presence of other polar or charged groups in the vicinity can also affect the likelihood of intramolecular hydrogen bonding. These groups can compete for hydrogen bonds or influence the conformation of the tyrosine side chain, affecting the hydrogen bonding potential.
Evidence and Examples
While directly observing intramolecular hydrogen bonds within a single tyrosine residue is challenging using standard experimental techniques, computational modeling and analyses of protein structures provide strong evidence for their existence under specific conditions.
Computational Studies: Molecular dynamics simulations and quantum mechanical calculations have shown that intramolecular hydrogen bonds in tyrosine side chains can form, but their stability and prevalence depend heavily on the factors mentioned above. These simulations can explore various conformations and quantify the strength of the hydrogen bond under different circumstances, offering valuable insights into their role in protein folding and stability.
Protein Structure Analysis: Examination of high-resolution protein structures determined through X-ray crystallography or NMR spectroscopy reveals instances where the tyrosine side chain forms intramolecular hydrogen bonds. However, these instances are not ubiquitous and are often context-dependent. The hydrogen bond may be present in one crystal structure but absent in another due to variations in the protein's conformation.
Implications for Protein Structure and Function:
The potential for intramolecular hydrogen bonding in tyrosine side chains has several implications for protein structure and function:
-
Protein Folding and Stability: Intramolecular hydrogen bonds can contribute to the stability of local secondary structures, such as turns and loops, within a protein. This contributes to the overall stability and proper folding of the protein.
-
Enzyme Activity: In some enzymes, the intramolecular hydrogen bonding of tyrosine residues can be crucial for maintaining the active site conformation and enabling catalytic activity.
-
Protein-Protein Interactions: Tyrosine residues involved in intermolecular interactions can influence the strength and specificity of these interactions. Intramolecular hydrogen bonding might affect the accessibility and orientation of the tyrosine side chain, thereby modulating the intermolecular interactions.
-
Post-translational modifications: Tyrosine can undergo various post-translational modifications, such as phosphorylation. The presence or absence of intramolecular hydrogen bonding could influence the susceptibility of tyrosine residues to these modifications.
Distinguishing Intramolecular from Intermolecular Hydrogen Bonds
It's crucial to differentiate between intramolecular hydrogen bonds (within a single tyrosine molecule) and intermolecular hydrogen bonds (between different molecules, including tyrosine and other molecules like water). In the context of proteins, intermolecular hydrogen bonds are far more common and often crucial for stabilizing the overall protein structure through interactions between different amino acid residues. While intramolecular hydrogen bonds can exist within the tyrosine side chain, their prevalence and impact are generally less significant than intermolecular interactions.
Factors influencing the probability of intramolecular H-bonding in tyrosine
Several factors affect the likelihood of observing intramolecular hydrogen bonding within a tyrosine residue:
-
The primary sequence: The arrangement of amino acids surrounding the tyrosine residue in the primary sequence can influence its local conformation and thus affect the probability of intramolecular hydrogen bond formation.
-
The secondary structure: Alpha-helices and beta-sheets, for example, have characteristic conformations that could make intramolecular hydrogen bonding either more or less likely.
-
The tertiary structure: The overall three-dimensional folding of the protein is the ultimate determinant of the spatial proximity of different parts of the molecule, thus affecting the probability of intramolecular hydrogen bonding.
Conclusion
In conclusion, while tyrosine's side chain can form intramolecular hydrogen bonds, this is not a guaranteed or consistently observed event. The occurrence of such a bond depends significantly on the protein's overall structure, the local environment around the tyrosine residue, and the competitive effects of solvent molecules and other potential hydrogen bond acceptors or donors. Computational and experimental studies are crucial for understanding the intricacies of these interactions and their role in protein structure, function, and dynamics. The rarity and context-dependency of intramolecular hydrogen bonds in tyrosine residues should not overshadow their potential impact on specific proteins and biological systems where they may play a crucial role. Further research is needed to fully elucidate the extent and significance of intramolecular hydrogen bonds involving tyrosine and other amino acids. This includes exploring new experimental techniques and enhancing computational models for more accurate prediction and understanding of these subtle but potentially critical molecular interactions.
Latest Posts
Latest Posts
-
Which Eukaryotic Cell Cycle Event Is Missing In Binary Fission
Mar 14, 2025
-
Countries With The Highest Degrees Of Government Bureaucratic Inefficiency Index
Mar 14, 2025
-
Complete Chemotherapy Orders Include A Second Identifier
Mar 14, 2025
-
At The Dawn Of Commercial Mobile Phone Technology At
Mar 14, 2025
-
Earth Science Tarbuck Lutgens 7th Edition
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Can Tyrosine Form Hydrogen Bonds With Its Side Chain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
