C3h6o I2 Goes To C3h5io I- H Activation Energy
Holbox
Mar 20, 2025 · 5 min read
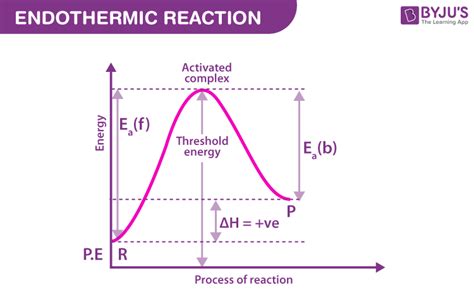
Table of Contents
C3H6O + I2 → C3H5IO + HI: Activation Energy and Reaction Mechanism
The reaction between propanal (C3H6O) and iodine (I2) to form 1-iodopropan-1-ol (C3H5IO) and hydrogen iodide (HI) is a fascinating example of a chemical transformation involving iodine's electrophilic properties and the reactivity of aldehydes. While the overall reaction appears straightforward, understanding the underlying mechanism, specifically the activation energy barrier, requires a detailed examination of the individual steps involved. This article delves into the intricacies of this reaction, focusing on the activation energy and exploring various aspects influencing its magnitude.
Understanding the Reaction: C3H6O + I2 → C3H5IO + HI
The reaction between propanal and iodine is not a simple one-step process. It's a multi-step reaction involving several intermediate species and transition states. The key to understanding the activation energy lies in dissecting this mechanism. A plausible mechanism, focusing on the role of iodine as an electrophile, is as follows:
Step 1: Electrophilic Attack by Iodine
The reaction initiates with the electrophilic attack of iodine (I2) on the carbonyl oxygen of propanal (C3H6O). The carbonyl oxygen, being relatively electron-rich due to the presence of the lone pairs, acts as a nucleophile. This interaction forms a polarized intermediate where the oxygen carries a partial positive charge and one iodine atom carries a partial negative charge. This step is crucial, as it determines the overall rate of the reaction.
I2 + C3H6O ⇌ [C3H6O-Iδ+…Iδ-] (Equilibrium step)
This equilibrium is established rapidly, and the concentration of the intermediate species is relatively low. The formation of this intermediate is reversible; the activation energy of this step represents the energy required to overcome the electrostatic repulsion between the iodine molecule and the carbonyl group.
Step 2: Nucleophilic Attack by Iodide Ion
The polarized iodine molecule then undergoes heterolytic cleavage, leading to the formation of an iodide ion (I⁻) and a positively charged iodinium ion. The iodide ion now acts as a nucleophile, attacking the electrophilic carbon of the carbonyl group. This is a relatively fast step, although it has its own activation energy barrier.
[C3H6O-Iδ+…Iδ-] → C3H6O-I⁺ + I⁻ (Fast Step)
C3H6O-I⁺ + I⁻ → C3H6(OI)I (Fast Step)
This step results in the formation of an unstable intermediate where both iodine and the hydroxyl group are bonded to the carbon atom. This intermediate is highly reactive.
Step 3: Proton Transfer and Product Formation
The next step involves a proton transfer from the hydroxyl group to the iodide ion. This proton transfer is facilitated by the presence of a solvent or possibly by another molecule of propanal or iodine.
C3H6(OI)I + I⁻ → C3H5IO + HI
This step leads to the formation of the final products, 1-iodopropan-1-ol (C3H5IO) and hydrogen iodide (HI). This is generally an exothermic step, meaning it releases energy. The overall reaction is also exothermic; this implies that the activation energy of the rate-determining step is higher than the exothermicity of the reaction.
Factors Affecting Activation Energy
Several factors can significantly influence the activation energy of this reaction:
1. Solvent Effects:
The solvent plays a crucial role in determining the activation energy. Polar solvents can stabilize the intermediate species, lowering the energy required to reach the transition state. Conversely, nonpolar solvents might hinder the reaction by destabilising the polar intermediate, thereby increasing the activation energy.
2. Temperature:
Temperature is directly proportional to the reaction rate. Higher temperatures provide molecules with more kinetic energy, increasing the probability of overcoming the activation energy barrier. The Arrhenius equation demonstrates this relationship: k = Ae^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature.
3. Presence of Catalysts:
The use of catalysts can significantly reduce the activation energy of the reaction. Catalysts provide an alternative reaction pathway with a lower activation energy barrier, thereby accelerating the reaction rate. For example, Lewis acids could potentially catalyze this reaction by coordinating to the carbonyl oxygen, making it a better nucleophile.
4. Steric Effects:
The steric hindrance around the carbonyl group in propanal could affect the rate of the reaction. Bulky substituents near the carbonyl group might hinder the approach of the iodine molecule, increasing the activation energy.
5. Iodine Concentration:
The concentration of iodine is directly proportional to the reaction rate. A higher iodine concentration leads to a higher probability of collision with propanal molecules, thus increasing the reaction rate by increasing the frequency of successful collisions capable of overcoming the activation energy barrier.
Determining Activation Energy Experimentally
The activation energy can be determined experimentally using various techniques, primarily involving measuring the reaction rate at different temperatures. The Arrhenius equation can then be used to plot ln(k) against 1/T. The slope of this plot is equal to -Ea/R, allowing for the calculation of the activation energy.
Conclusion
The reaction between propanal and iodine is a complex multi-step process involving several intermediate species and transition states. The activation energy for this reaction is influenced by various factors, including the solvent used, temperature, presence of catalysts, steric effects, and the concentration of iodine. Understanding the reaction mechanism and the factors influencing the activation energy is crucial for controlling and optimizing this type of reaction for synthetic purposes or in understanding related chemical transformations. Further research into this reaction could involve studying the precise nature of the intermediate species, the role of specific solvents, and exploring the potential for catalysis to further reduce the activation energy and improve efficiency. This knowledge could have implications in various fields, including organic synthesis and chemical kinetics. Advanced computational techniques such as density functional theory (DFT) calculations can offer valuable insights into the energy profiles of this reaction, providing a more quantitative understanding of the activation energy and the overall reaction pathway.
Latest Posts
Latest Posts
-
Which Statement About Groupthink Is Correct
Mar 20, 2025
-
The Building Blocks Of Nucleic Acids Are
Mar 20, 2025
-
To Avoid Fatigue When Should Team Roles
Mar 20, 2025
-
Which Sentence Uses The Underlined Word Correctly
Mar 20, 2025
-
The Great Compromise Did All Of The Following Except
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about C3h6o I2 Goes To C3h5io I- H Activation Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
