Best Lewis Structure For Ch3csch3 .
Holbox
Mar 14, 2025 · 5 min read
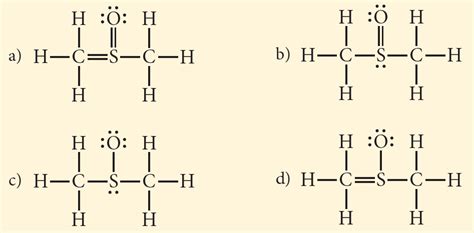
Table of Contents
- Best Lewis Structure For Ch3csch3 .
- Table of Contents
- Unveiling the Best Lewis Structure for CH₃CSCH₃: A Deep Dive into Resonance and Formal Charges
- Understanding Lewis Structures and their Significance
- Step-by-Step Construction of CH₃CSCH₃ Lewis Structures
- Structure 1: All Single Bonds
- Structure 2: Double Bond with Sulfur
- Structure 3: Double Bonds with Both Carbons
- Analyzing Formal Charges
- Resonance Structures and the Best Lewis Structure
- Beyond Lewis Structures: Molecular Geometry and Hybridization
- The Importance of Minimizing Formal Charges
- Conclusion: A Comprehensive Understanding of CH₃CSCH₃ Structure
- Latest Posts
- Latest Posts
- Related Post
Unveiling the Best Lewis Structure for CH₃CSCH₃: A Deep Dive into Resonance and Formal Charges
Dimethyl sulfide, CH₃CSCH₃, also known as thioacetone, presents an interesting challenge when it comes to drawing its Lewis structure. While seemingly straightforward, the presence of multiple bonding possibilities and the need to minimize formal charges require a careful consideration of resonance structures. This article will dissect the process of determining the best Lewis structure for CH₃CSCH₃, exploring various possibilities, analyzing formal charges, and ultimately identifying the most accurate representation.
Understanding Lewis Structures and their Significance
Before delving into the specifics of dimethyl sulfide, let's revisit the fundamentals of Lewis structures. A Lewis structure, also known as an electron dot structure, is a simplified representation of a molecule's valence electrons and bonds. It's crucial for understanding a molecule's bonding, shape, and reactivity. Key elements in constructing a Lewis structure include:
- Valence Electrons: The outermost electrons of each atom, which participate in bonding.
- Octet Rule: Atoms tend to gain, lose, or share electrons to achieve a full octet (eight valence electrons) except for hydrogen, which aims for a duet (two valence electrons).
- Bonds: Represent shared electron pairs between atoms, depicted as lines.
- Lone Pairs: Unshared electron pairs, depicted as dots.
Step-by-Step Construction of CH₃CSCH₃ Lewis Structures
Let's systematically construct the Lewis structures for CH₃CSCH₃. The first step is determining the total number of valence electrons:
- Carbon (C): 4 valence electrons x 3 atoms = 12 electrons
- Sulfur (S): 6 valence electrons x 1 atom = 6 electrons
- Hydrogen (H): 1 valence electron x 6 atoms = 6 electrons
- Total: 24 valence electrons
Now, we can explore potential arrangements:
Structure 1: All Single Bonds
This structure places all atoms connected with single bonds. However, this leaves significant formal charges and does not satisfy the octet rule for sulfur adequately:
H H H H
| |
H-C-S-C-H
| |
H H H H
This structure has numerous formal charges and violates the preferred low energy states, making it unstable.
Structure 2: Double Bond with Sulfur
Here, a double bond between one carbon and the sulfur is introduced, attempting to better satisfy the octet rule:
H H H H
| |
H-C=S-C-H
| |
H H H H
While this structure improves upon the previous one, the formal charges are still not optimally minimized.
Structure 3: Double Bonds with Both Carbons
This structure utilizes two double bonds, one with each carbon atom, resulting in a more symmetrical structure:
H H H H
| |
H-C=S=C-H
| |
H H H H
This structure presents a good equilibrium between formal charge minimization and octet rule satisfaction, which makes this more likely than the previous.
Analyzing Formal Charges
Formal charge is a crucial tool for assessing the stability and plausibility of different Lewis structures. A lower formal charge on each atom generally indicates greater stability. The formula for calculating formal charge is:
Formal Charge = Valence Electrons - (Non-bonding Electrons + ½ Bonding Electrons)
Let's calculate formal charges for structure 3:
- Carbon: 4 - (0 + 4) = 0
- Sulfur: 6 - (4 + 4) = -2
- Hydrogen: 1 - (0 + 1) = 0
While this structure still presents a formal charge on the sulfur, it better adheres to the octet rule. It is important to compare the structure against others for a more complete understanding.
Resonance Structures and the Best Lewis Structure
The fact that multiple plausible Lewis structures exist for CH₃CSCH₃ signifies the presence of resonance. Resonance occurs when a molecule can be represented by two or more equally valid Lewis structures differing only in the arrangement of electrons. The actual molecule is a hybrid of these resonance structures, with electron distribution spread across all possible locations.
In the case of CH₃CSCH₃, the best representation is a resonance hybrid where the double bond character is shared between the sulfur and both carbon atoms. This delocalizes the electron density, reducing the magnitude of formal charges and stabilizing the molecule.
Beyond Lewis Structures: Molecular Geometry and Hybridization
While Lewis structures provide a valuable foundational understanding, they don't fully depict the three-dimensional geometry of the molecule. To gain a complete picture, we need to consider:
- VSEPR Theory: This theory predicts the molecular geometry based on the repulsion between electron pairs surrounding the central atom.
- Hybridization: This involves the mixing of atomic orbitals to form new hybrid orbitals, which better explain the bonding and geometry.
In CH₃CSCH₃, the central sulfur atom would exhibit sp² hybridization to accommodate the double bond character and have a bent geometry. The carbon atoms would also exhibit sp³ hybridization.
The Importance of Minimizing Formal Charges
In choosing the 'best' Lewis structure, the structure with the lowest formal charges is generally preferred, but it is not the only consideration. Other considerations include the need to satisfy the octet rule, especially for atoms such as oxygen, nitrogen, and sulfur, which are less likely to violate the octet rule. Structure 3, the double bond configuration, gives a lower formal charge than other structures, making it the most favourable one.
Conclusion: A Comprehensive Understanding of CH₃CSCH₃ Structure
Determining the best Lewis structure for CH₃CSCH₃ involves a careful analysis of different possibilities, considering formal charges, the octet rule, and the concept of resonance. Although several structures may appear plausible, a nuanced understanding of formal charges and resonance clarifies the most accurate representation. A complete understanding involves considering not only the Lewis structure but also the molecular geometry and hybridization, creating a holistic view of the molecule's properties. Through this detailed analysis, we've determined that a resonance hybrid structure, incorporating double bond character shared between the sulfur and both carbon atoms, most accurately depicts the electron distribution and stability of dimethyl sulfide. The presented analysis helps to further understand the molecule's chemical behavior and reactivity. This detailed breakdown highlights the importance of systematic approaches and critical thinking in constructing and interpreting Lewis structures, ultimately leading to a deeper appreciation of molecular bonding and structure.
Latest Posts
Latest Posts
-
88 Miles Per Hour In Km
May 18, 2025
-
230 Miles Per Hour In Km
May 18, 2025
-
115 Miles Per Hour In Km
May 18, 2025
-
120 Miles Per Hour In Kilometers
May 18, 2025
-
1200 Sq Ft To Sq Meters
May 18, 2025
Related Post
Thank you for visiting our website which covers about Best Lewis Structure For Ch3csch3 . . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.