Benzoic Acid + Ch3ch2cl + Alcl3
Holbox
May 10, 2025 · 5 min read
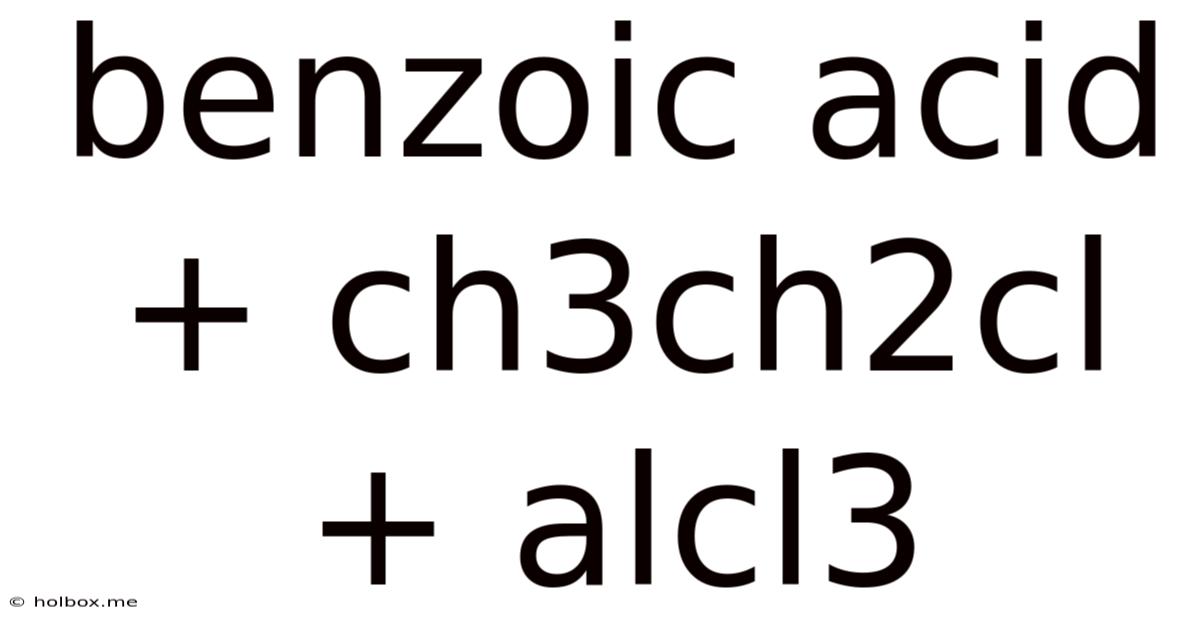
Table of Contents
Friedel-Crafts Alkylation: A Deep Dive into Benzoic Acid, Chloroethane, and Aluminum Chloride
The reaction between benzoic acid, chloroethane (ethyl chloride), and aluminum chloride (AlCl₃) presents a fascinating case study in organic chemistry, specifically concerning Friedel-Crafts alkylation. While seemingly straightforward, this reaction reveals complexities and limitations frequently encountered in this powerful yet sometimes unpredictable reaction class. This article will delve into the mechanism, challenges, and potential outcomes of this specific reaction, exploring the nuances that make it a rich topic for study.
Understanding Friedel-Crafts Alkylation
Friedel-Crafts alkylation is a cornerstone reaction in organic synthesis, enabling the introduction of alkyl groups onto aromatic rings. It typically involves an alkyl halide (like chloroethane in our example) and a Lewis acid catalyst, most commonly aluminum chloride (AlCl₃). The Lewis acid plays a crucial role in activating the alkyl halide, making it a better electrophile and thus more susceptible to attack by the aromatic ring.
The General Mechanism:
-
Formation of the Electrophile: The Lewis acid (AlCl₃) coordinates with the alkyl halide (CH₃CH₂Cl), forming a complex that weakens the C-Cl bond. This makes the carbon atom of the alkyl group more electrophilic. The chloride ion then departs, leaving behind a carbocation (CH₃CH₂⁺). This step is crucial and can be significantly influenced by the structure of the alkyl halide.
-
Electrophilic Aromatic Substitution: The electrophilic carbocation attacks the electron-rich aromatic ring of the benzene derivative. This attack leads to the formation of a resonance-stabilized carbocation intermediate.
-
Deprotonation: A base (often the conjugate base of the Lewis acid, AlCl₄⁻) abstracts a proton from the positively charged intermediate, restoring aromaticity and completing the alkylation.
The Complication: Benzoic Acid as a Substrate
The presence of benzoic acid introduces significant challenges to a straightforward Friedel-Crafts alkylation. Benzoic acid possesses a carboxyl group (-COOH), which is strongly electron-withdrawing. This electron-withdrawing effect significantly deactivates the benzene ring towards electrophilic aromatic substitution. This deactivation arises because the carboxyl group pulls electron density away from the ring, making it less nucleophilic and less likely to be attacked by the electrophile.
Deactivation and Directing Effects:
The carboxyl group is a meta-directing group. This means that if alkylation were to occur, it would preferentially occur at the meta position relative to the carboxyl group. However, the significant deactivation often prevents the reaction from proceeding at all under typical Friedel-Crafts conditions.
Competing Reactions:
The presence of the acidic carboxyl group also creates the possibility of competing reactions. The highly reactive carbocation intermediate, CH₃CH₂⁺, could potentially react with the carboxyl group itself, leading to side reactions and lower yields of the desired alkylated product. This side reaction could involve esterification or other complex transformations, further hindering the formation of the expected product.
Potential Outcomes and Modifications
Given the strong deactivating effect of the carboxyl group, a direct Friedel-Crafts alkylation of benzoic acid with chloroethane and AlCl₃ is highly unlikely to yield the desired product in appreciable amounts. The reaction might proceed very slowly, if at all, with low yields and a complex mixture of side products.
Possible Modifications and Alternative Strategies:
Several strategies could be employed to circumvent the challenges posed by the benzoic acid substrate:
-
Protecting Group Strategy: One approach would be to temporarily protect the carboxyl group, converting it into a less reactive derivative before performing the Friedel-Crafts alkylation. Common protecting groups for carboxylic acids include esters (e.g., methyl ester) or amides. After the alkylation, the protecting group could be removed, regenerating the carboxylic acid functionality. This approach effectively removes the deactivating and steric hindrance of the -COOH group.
-
Alternative Alkylating Agents: Exploring alternative alkylating agents with higher reactivity might improve the chances of alkylation, although it’s unlikely to overcome the fundamental issue of the deactivated aromatic ring.
-
Different Lewis Acid Catalysts: While AlCl₃ is the most common catalyst, exploring other Lewis acids might offer slight advantages, although significant improvement is doubtful.
-
Changing Reaction Conditions: Optimizing reaction parameters like temperature, solvent, and reaction time could potentially improve the yield, but it's unlikely to overcome the inherent challenges of the reaction.
-
Alternative Synthetic Routes: Considering alternative routes that don't rely on Friedel-Crafts alkylation would likely be the most efficient method. These could involve methods such as:
- Grignard Reaction followed by Carbonation: A Grignard reagent could be prepared from an alkyl halide, then reacted with carbon dioxide to introduce the carboxyl group.
- Diazonium Salt Reaction: This versatile method enables functionalization of aromatic rings, providing a possible route to introduce the desired alkyl group.
Conclusion
The reaction of benzoic acid, chloroethane, and aluminum chloride highlights the importance of understanding the interplay between substrate structure, reaction mechanisms, and reaction conditions in organic synthesis. The strong deactivating and meta-directing influence of the carboxyl group significantly hampers a direct Friedel-Crafts alkylation approach. While minor modifications to the reaction conditions might be attempted, alternative synthetic strategies, such as protecting group chemistry or entirely different synthetic routes, are far more likely to yield the desired alkylated product. This case study underscores the value of careful consideration of reactivity and strategic planning in the design and execution of organic syntheses. It showcases how seemingly straightforward reactions can present unexpected complexities and the importance of adapting synthetic strategies based on the unique challenges of specific substrates. Furthermore, it emphasizes the need for a deep understanding of reaction mechanisms and the ability to predict potential pitfalls and devise solutions based on a solid theoretical foundation.
Latest Posts
Related Post
Thank you for visiting our website which covers about Benzoic Acid + Ch3ch2cl + Alcl3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.