All Organic Compounds Contain: Question 7 Options: Oxygen Hydrogen Carbon
Holbox
Mar 24, 2025 · 5 min read
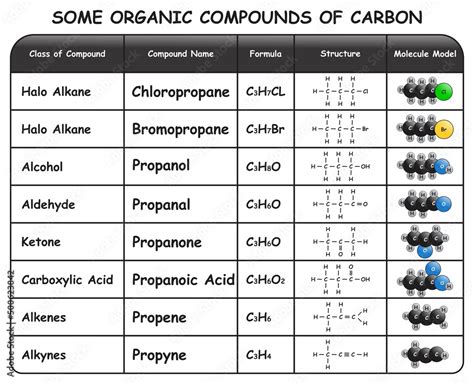
Table of Contents
- All Organic Compounds Contain: Question 7 Options: Oxygen Hydrogen Carbon
- Table of Contents
- All Organic Compounds Contain: Carbon
- The Uniqueness of Carbon: The Building Block of Life
- Tetravalency: The Foundation of Carbon's Versatility
- Catentation: The Ability to Form Chains and Rings
- Isomerism: The Multiplicity of Molecular Structures
- Hybridization: The Shaping of Molecular Geometry
- The Roles of Oxygen and Hydrogen in Organic Compounds
- Oxygen: Functional Groups and Reactivity
- Hydrogen: Structure and Bonding
- Exceptions and Challenges: Organometallic Compounds and Other Considerations
- Conclusion: Carbon – The Indispensable Element
- Latest Posts
- Latest Posts
- Related Post
All Organic Compounds Contain: Carbon
The question, "All organic compounds contain: Oxygen, Hydrogen, Carbon," presents a fundamental concept in organic chemistry. While many organic compounds do contain oxygen and hydrogen, the definitive answer is carbon. Let's delve into the reasons why, exploring the characteristics of carbon that make it the cornerstone of organic chemistry and examining the roles of oxygen and hydrogen within this vast and complex field.
The Uniqueness of Carbon: The Building Block of Life
Carbon's unique properties make it the fundamental element of organic chemistry. These properties allow for the formation of an incredibly diverse range of molecules, forming the basis of all known life forms and a vast array of synthetic materials. Let's explore what sets carbon apart:
Tetravalency: The Foundation of Carbon's Versatility
Carbon possesses four valence electrons, meaning it can form four covalent bonds with other atoms. This tetravalency is the key to carbon's ability to create complex and diverse structures. Unlike many other elements, carbon can form strong bonds not only with itself but also with a wide range of other atoms, including hydrogen, oxygen, nitrogen, sulfur, phosphorus, and halogens. This allows for the construction of long chains, branched structures, rings, and intricate three-dimensional frameworks.
Catentation: The Ability to Form Chains and Rings
Carbon's ability to form strong bonds with other carbon atoms is known as catenation. This remarkable property allows carbon atoms to link together to form long chains, branched structures, and rings, the building blocks of many organic molecules. The length and arrangement of these carbon chains and rings are crucial in determining the properties and functions of organic compounds. The limitless possibilities for carbon-carbon bonding are what drives the immense diversity seen in organic molecules.
Isomerism: The Multiplicity of Molecular Structures
The ability of carbon to form multiple bonds (single, double, and triple bonds) and to arrange itself in various three-dimensional structures leads to the phenomenon of isomerism. Isomers are molecules with the same molecular formula but different structural arrangements. This means that two molecules can have the same number and types of atoms but have vastly different properties due to their distinct structures. This structural diversity further expands the already enormous potential for carbon to form an incredibly wide range of molecules.
Hybridization: The Shaping of Molecular Geometry
Carbon's ability to undergo hybridization is another critical factor in its versatility. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. The most common types of hybridization in carbon are sp, sp², and sp³. These hybrid orbitals influence the bond angles and overall geometry of organic molecules, impacting their physical and chemical properties. For example, the sp³ hybridization leads to tetrahedral geometry in methane (CH₄), while sp² hybridization results in the trigonal planar geometry in ethene (C₂H₄).
The Roles of Oxygen and Hydrogen in Organic Compounds
While carbon is the essential element defining organic chemistry, oxygen and hydrogen are extremely prevalent and play crucial roles in the structure and function of many organic molecules.
Oxygen: Functional Groups and Reactivity
Oxygen frequently appears in organic compounds, often as part of functional groups. Functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical reactions. Oxygen is found in many important functional groups such as:
- Hydroxyl (-OH): Found in alcohols, which are crucial solvents and have diverse applications.
- Carbonyl (C=O): Found in aldehydes, ketones, carboxylic acids, and esters, all possessing distinct reactivity. Aldehydes and ketones are important in fragrances and flavors, while carboxylic acids are essential in biological processes and the synthesis of polymers. Esters are commonly used as solvents and flavoring agents.
- Ether (-O-): Ethers are used as solvents and anesthetics.
- Peroxide (-O-O-): Peroxides are strong oxidizing agents used in various chemical processes.
The presence of oxygen significantly influences the polarity and reactivity of organic molecules, making them suitable for a wide range of applications and biological functions.
Hydrogen: Structure and Bonding
Hydrogen is the most abundant element in organic compounds. It is primarily involved in forming single covalent bonds with carbon atoms and other elements like oxygen and nitrogen. Hydrogen's small size allows it to participate in diverse bonding arrangements, influencing the overall shape and properties of organic molecules. The number of hydrogen atoms attached to a carbon atom significantly influences the molecule's properties. For example, the presence of more hydrogen atoms generally leads to increased hydrophobicity (water-repelling property) in a molecule.
Exceptions and Challenges: Organometallic Compounds and Other Considerations
While carbon is the defining element of organic chemistry, the strict definition of "organic" has evolved over time. The development of organometallic chemistry, which involves compounds containing carbon-metal bonds, has blurred the lines somewhat. These compounds exhibit properties and reactivity that bridge the gap between traditional organic and inorganic chemistry. However, even in organometallic compounds, carbon remains central to the molecule’s structure and functionality.
Furthermore, there are a few exceptions to the general rule that all organic compounds contain carbon. Some simple carbon-free compounds, such as carbon dioxide (CO₂) and carbonates, were historically considered inorganic but are now sometimes included in the broader context of organic chemistry. This underscores the fluid and evolving nature of chemical classifications.
Conclusion: Carbon – The Indispensable Element
In summary, while oxygen and hydrogen are abundant and important constituents in many organic compounds, carbon remains the defining element. Its unique properties—tetravalency, catenation, isomerism, and hybridization—allow for the formation of the vast and diverse array of molecules that constitute the field of organic chemistry. The structural diversity arising from carbon's bonding capabilities is the driving force behind the complexity and significance of organic compounds in the natural world and in countless applications. The presence of oxygen and hydrogen contributes significantly to the reactivity and functionality of these molecules, but without carbon, the field of organic chemistry as we know it simply would not exist.
Latest Posts
Latest Posts
-
Campaigning Its A Process Answer Key
Mar 26, 2025
-
Rn Pharmacology Online Practice 2023 B
Mar 26, 2025
-
A Sales Rep Is Displaying His Companys Newest Smartwatches
Mar 26, 2025
-
Consider The Following Scenarios Which Behaviors Must Be Reported
Mar 26, 2025
-
Which Of The Following Is Correct
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about All Organic Compounds Contain: Question 7 Options: Oxygen Hydrogen Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
