All Isotopes Of Oxygen Must Have
Holbox
Mar 19, 2025 · 5 min read
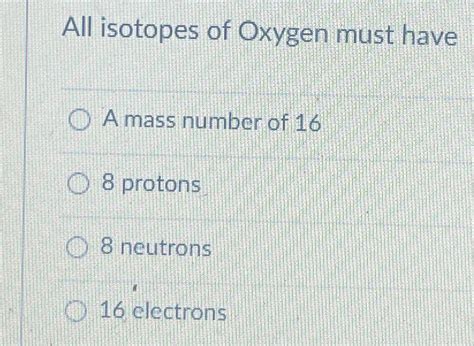
Table of Contents
All Isotopes of Oxygen Must Have: A Deep Dive into Oxygen's Atomic Structure and Properties
Oxygen, the life-giving element, is crucial for respiration and numerous other biological processes. But beyond its fundamental role in sustaining life, oxygen boasts a fascinating atomic structure, characterized by the existence of multiple isotopes. Understanding these isotopes and their common characteristics is essential for comprehending oxygen's behavior in various environments and its significance across diverse scientific fields. This comprehensive article will delve into the defining features that all isotopes of oxygen must possess, exploring their similarities and subtle differences.
The Defining Characteristics of All Oxygen Isotopes
All isotopes of oxygen, regardless of their mass number, share fundamental characteristics that define their elemental identity. These include:
1. The Same Number of Protons: Atomic Number 8
The most crucial characteristic defining all oxygen isotopes is their atomic number, which is 8. This means that every atom classified as oxygen possesses eight protons in its nucleus. The number of protons determines the element's identity; changing the proton count transforms the atom into a different element altogether. This fundamental property remains consistent across all oxygen isotopes.
2. The Same Number of Electrons: Maintaining Electrical Neutrality
In a neutral oxygen atom, the number of electrons orbiting the nucleus equals the number of protons. Therefore, all oxygen isotopes, in their neutral state, have eight electrons. This electron configuration determines oxygen's chemical properties, its reactivity, and its ability to form chemical bonds. The electron arrangement dictates the oxygen atom's tendency to gain two electrons to achieve a stable octet, forming the characteristic -2 oxidation state common in many oxygen compounds.
3. Similar Chemical Behavior: Driven by Electron Configuration
Despite variations in neutron number (discussed below), all oxygen isotopes exhibit similar chemical behavior. This similarity stems from their identical electron configuration. Chemical reactions are primarily governed by the interaction of electrons, and since all oxygen isotopes have the same number of electrons, they participate in similar chemical reactions and form analogous compounds. However, subtle differences in reaction rates and isotopic fractionation can occur due to mass differences, a topic explored later.
4. A Common Element Symbol: "O"
All oxygen isotopes are represented by the same chemical symbol, "O", in the periodic table. This shared symbol reflects their fundamental identity as oxygen atoms, irrespective of their mass number variations. This symbol serves as a concise and universally recognized representation for all isotopic forms of this crucial element.
Oxygen Isotopes: Variations in Neutron Number
While all oxygen isotopes share the defining characteristics mentioned above, they differ in their number of neutrons. The number of neutrons in an atom's nucleus determines its mass number, which is the sum of protons and neutrons. Oxygen's three main stable isotopes showcase this variation:
-
Oxygen-16 (¹⁶O): This is the most abundant isotope of oxygen, comprising approximately 99.76% of naturally occurring oxygen. It has 8 protons and 8 neutrons (16 - 8 = 8).
-
Oxygen-17 (¹⁷O): This isotope has 8 protons and 9 neutrons. It's present in much lower abundance (0.04%) compared to ¹⁶O.
-
Oxygen-18 (¹⁸O): This isotope contains 8 protons and 10 neutrons. Its natural abundance is around 0.20%.
Beyond these three stable isotopes, several radioactive oxygen isotopes exist, which are unstable and undergo radioactive decay. These radioactive isotopes have varying half-lives and decay mechanisms. The unstable isotopes have a different number of neutrons than the stable ones, leading to nuclear instability.
The Impact of Isotopic Variations: Mass-Dependent Fractionation
Although all oxygen isotopes exhibit similar chemical behavior, the differences in their masses lead to isotope fractionation. This phenomenon refers to the preferential enrichment or depletion of certain isotopes during various physical, chemical, and biological processes. Isotope fractionation is mass-dependent, meaning that heavier isotopes (like ¹⁸O) tend to react or move slightly slower than lighter isotopes (like ¹⁶O).
Examples of Isotope Fractionation:
-
Evaporation: During evaporation from water bodies, the lighter ¹⁶O evaporates more readily than ¹⁸O, leading to a slight enrichment of ¹⁸O in the remaining liquid water.
-
Photosynthesis: Plants preferentially incorporate ¹⁶O into their organic matter during photosynthesis, resulting in a lower ¹⁸O/¹⁶O ratio in plant tissues compared to the surrounding water.
-
Mineral Formation: The ¹⁸O/¹⁶O ratio in minerals can vary depending on the temperature and conditions under which they formed. This variation allows scientists to deduce information about past climates and geological processes.
These variations in isotopic ratios are exploited in diverse scientific disciplines, such as:
-
Paleoclimatology: Analyzing the ¹⁸O/¹⁶O ratio in ice cores and sediments helps reconstruct past climate conditions.
-
Hydrology: Tracing water sources and movement using isotopic signatures.
-
Geochemistry: Understanding mineral formation and geological processes.
-
Archaeology: Dating artifacts and studying ancient diets.
Radioactive Oxygen Isotopes: Decay and Applications
Several radioactive oxygen isotopes exist, each with unique decay properties and applications. These isotopes are primarily used in scientific research and medical applications, such as:
-
Oxygen-15 (¹⁵O): This positron-emitting isotope is used in positron emission tomography (PET) scans to visualize metabolic activity in the body. Its short half-life necessitates on-site production.
-
Oxygen-19 (¹⁹O): This isotope decays via beta emission and has applications in nuclear medicine and other research areas.
Conclusion: Unity in Diversity
All isotopes of oxygen share a common atomic number (8) and thus maintain the fundamental characteristics of oxygen. The number of electrons remains constant, leading to similar chemical behavior. However, variations in neutron numbers lead to isotopic mass differences, resulting in observable effects like mass-dependent isotope fractionation. This fractionation plays a crucial role in various geological, biological, and chemical processes. Understanding both the commonalities and differences among oxygen isotopes provides invaluable insights into Earth's systems, past climates, and diverse biological mechanisms. The study of oxygen isotopes serves as a powerful tool across a vast range of scientific disciplines, underscoring the multifaceted nature of this seemingly simple element. The exploration of oxygen's isotopic variations continues to deepen our understanding of the natural world and unlock new applications in science and technology.
Latest Posts
Latest Posts
-
John Is Rollerblading Down A Long
Mar 19, 2025
-
Hydrolysis Of Sucrose A Disaccharide Results In
Mar 19, 2025
-
What Is The Difference Between Mutualism And Synergism
Mar 19, 2025
-
The Supply Of A Good Will Be More Elastic The
Mar 19, 2025
-
What Do Economists Mean When They Say Behavior Is Rational
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about All Isotopes Of Oxygen Must Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
