A Clinical Trial Was Conducted To Test The Effectiveness
Holbox
Mar 27, 2025 · 6 min read
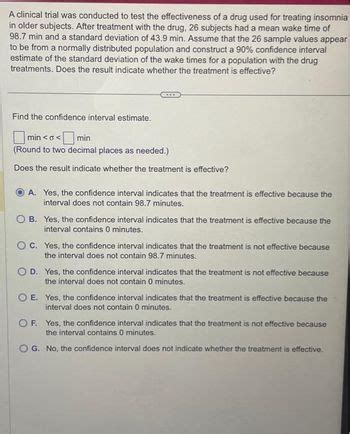
Table of Contents
- A Clinical Trial Was Conducted To Test The Effectiveness
- Table of Contents
- A Clinical Trial Was Conducted to Test the Effectiveness: Unveiling the Process and its Significance
- Understanding the Phases of a Clinical Trial
- Phase 1: Safety First
- Phase 2: Efficacy and Safety Assessment
- Phase 3: Confirmation of Efficacy and Monitoring Side Effects
- Phase 4: Post-Market Surveillance
- The Importance of Ethical Considerations
- Data Analysis and Interpretation: Unveiling the Truth
- The Impact of Clinical Trials on Healthcare
- The Future of Clinical Trials
- Conclusion: A Cornerstone of Medical Progress
- Latest Posts
- Latest Posts
- Related Post
A Clinical Trial Was Conducted to Test the Effectiveness: Unveiling the Process and its Significance
Conducting a clinical trial to test the effectiveness of a new treatment, drug, or device is a complex, multi-stage process crucial for advancing healthcare. This article delves deep into the intricacies of clinical trials, exploring their phases, ethical considerations, data analysis, and ultimate impact on patient care. We'll examine the rigorous methodology employed to ensure the reliability and validity of the results, highlighting the significance of these trials in bringing safe and effective medical interventions to the public.
Understanding the Phases of a Clinical Trial
Clinical trials are meticulously structured, typically divided into four phases, each with specific objectives:
Phase 1: Safety First
The primary focus of Phase 1 trials is safety. A small group of healthy volunteers (usually 20-100 participants) receive the experimental treatment to determine its safety profile, identify potential side effects, and establish a safe dosage range. Pharmacokinetics (how the body processes the drug) and pharmacodynamics (how the drug affects the body) are also assessed during this crucial phase. This is a foundational step, ensuring the treatment is not inherently harmful before moving to larger-scale studies. Data collection during this phase is rigorous, meticulously documenting any adverse events and physiological responses.
Phase 2: Efficacy and Safety Assessment
Phase 2 trials expand the participant pool (typically 100-300 individuals) to evaluate the treatment's efficacy and further assess its safety. Participants in this phase usually have the condition the treatment is intended to address. The focus shifts from mere safety to observing whether the treatment shows promise in achieving its intended therapeutic effect. Different dosages may be tested to optimize effectiveness and minimize adverse effects. Statistical analysis begins to play a more significant role, helping researchers understand the treatment's potential benefits compared to a placebo or standard treatment.
Phase 3: Confirmation of Efficacy and Monitoring Side Effects
Phase 3 trials are the most extensive, involving hundreds or even thousands of participants across multiple locations. This phase aims to confirm the treatment's efficacy, compare it against existing treatments (if any), and thoroughly monitor for both common and rare side effects. The large sample size enhances the statistical power, providing a more reliable estimate of the treatment's effectiveness and risks in a real-world setting. Rigorous data management and blinded studies (where participants and researchers don't know who's receiving the experimental treatment versus a placebo) are crucial to minimizing bias and ensuring accurate results. This phase is pivotal in determining whether the treatment is genuinely effective and warrants regulatory approval.
Phase 4: Post-Market Surveillance
Even after receiving regulatory approval, the treatment's journey isn't over. Phase 4 trials, also known as post-market surveillance, involve monitoring the treatment's long-term effects and safety in a broader population after it's released commercially. This long-term observation helps identify rare or delayed side effects that might not have been apparent during earlier phases. The data collected informs ongoing safety monitoring and may lead to updates in prescribing information or even the withdrawal of the treatment if significant safety concerns arise. This phase underscores the commitment to ongoing patient safety and the continuous refinement of medical knowledge.
The Importance of Ethical Considerations
Clinical trials must adhere to strict ethical guidelines to protect the rights and welfare of participants. Informed consent is paramount – potential participants must be fully informed about the trial's purpose, procedures, potential risks and benefits, and their right to withdraw at any time without penalty. Independent ethics committees review all aspects of the trial protocol to ensure it aligns with ethical standards. Confidentiality and data privacy are strictly enforced throughout the process. The ethical conduct of clinical trials is non-negotiable, underpinning the integrity of the research and maintaining public trust.
Data Analysis and Interpretation: Unveiling the Truth
The data collected during each phase undergoes rigorous statistical analysis. This involves sophisticated techniques to assess the treatment's effectiveness, compare it to existing therapies, and identify any adverse events. Statistical significance plays a crucial role in determining whether observed results are likely due to the treatment or mere chance. Confidence intervals and p-values are key metrics used to quantify the certainty of the findings. The interpretation of results requires careful consideration of various factors, including sample size, study design, and the presence of any potential biases. Transparency and reproducibility are cornerstones of credible clinical trial research.
The Impact of Clinical Trials on Healthcare
The impact of successful clinical trials on healthcare is profound and far-reaching. They lead to the development of:
- New and Improved Treatments: Clinical trials pave the way for innovative treatments for a vast array of diseases, significantly improving patient outcomes and quality of life.
- Enhanced Safety Standards: The rigorous safety assessments in clinical trials ensure that new therapies are safe for use in the general population, minimizing the risk of harm.
- Informed Healthcare Decisions: The evidence generated by clinical trials provides healthcare professionals with the necessary information to make informed decisions about treatment options for their patients.
- Improved Diagnostic Tools: Clinical trials also evaluate new diagnostic techniques, leading to more accurate and efficient disease detection.
- Public Health Advancement: Clinical trials often contribute significantly to advancing public health initiatives, leading to improved prevention strategies and better health outcomes for entire populations.
The Future of Clinical Trials
Technological advancements are transforming the landscape of clinical trials. Digital technologies such as telemedicine, wearable sensors, and electronic data capture systems are streamlining the process, improving data collection efficiency, and facilitating remote participation. Artificial intelligence (AI) and machine learning (ML) are playing increasingly important roles in analyzing large datasets, identifying patterns, and accelerating the development of new treatments. The future of clinical trials promises to be faster, more efficient, and more inclusive, leading to a wider availability of life-changing treatments.
Conclusion: A Cornerstone of Medical Progress
Clinical trials are the cornerstone of medical progress, representing a vital pathway for bringing safe and effective treatments to patients. The rigorous methodology, ethical considerations, and detailed data analysis ensure the reliability and validity of the findings. This multi-stage process, from initial safety assessments to post-market surveillance, underscores the commitment to developing and providing high-quality healthcare. The future of clinical trials, driven by technological innovation, promises to even further enhance the efficiency and effectiveness of medical research, ultimately benefiting patients around the world. Understanding the intricacies of clinical trials not only enhances public appreciation of medical advancement but also contributes to informed healthcare discussions and the ongoing pursuit of better health outcomes for all. The significance of these trials cannot be overstated; they are the bedrock of modern medicine and a critical driver of improved global health.
Latest Posts
Latest Posts
-
When Can A Notary Submit An Application For Reappointment
Mar 31, 2025
-
A Financial Advisor Schedules An Introductory Meeting
Mar 31, 2025
-
Match Each Principal Function Of Management With Its Definition
Mar 31, 2025
-
Draw The Major Organic Product Of The Following Reaction
Mar 31, 2025
-
Which Of The Following Statements About Variation Is False
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about A Clinical Trial Was Conducted To Test The Effectiveness . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
