A 3d Representation Of A Cyclohexane
Holbox
Mar 18, 2025 · 5 min read
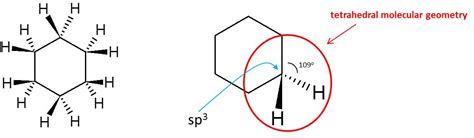
Table of Contents
A 3D Representation of Cyclohexane: Conformations, Stability, and Applications
Cyclohexane, a seemingly simple molecule with the chemical formula C₆H₁₂, holds a fascinating complexity when its three-dimensional structure is considered. Understanding its various conformations, their relative stabilities, and the implications for its chemical reactivity is crucial in organic chemistry. This article delves into the intricacies of cyclohexane's 3D representation, exploring its different conformations, analyzing their energy differences, and highlighting its significance in various fields.
Chair Conformation: The Most Stable Form
The most stable conformation of cyclohexane is the chair conformation. This structure minimizes steric strain, a crucial factor in determining molecular stability. Imagine a chair with its back and seat; this is analogous to the cyclohexane ring.
Understanding the Chair Conformation's Features:
-
Axial and Equatorial Positions: Each carbon atom in the chair conformation has two bonds: one pointing straight up or down (axial) and one pointing outward, roughly parallel to the plane of the ring (equatorial). The alternating axial and equatorial positions are a key characteristic that minimizes steric hindrance.
-
Steric Strain Minimization: The chair conformation is favored due to the staggered arrangement of neighboring hydrogen atoms. This staggered arrangement reduces steric interactions between bulky substituents, leading to a lower energy state compared to other conformations.
-
Visualization Techniques: To visualize the chair conformation effectively, consider using molecular modeling software or building physical models. These tools help understand the three-dimensional relationships between atoms and bonds.
Energy Difference between Axial and Equatorial Substituents:
While the chair conformation itself is the most stable, placing substituents on the cyclohexane ring introduces energy differences based on their axial or equatorial positions. Bulky substituents prefer the equatorial position, which places them further away from other atoms, thus minimizing steric interactions.
-
A-Values: The energy difference between axial and equatorial positions for a given substituent is represented by its A-value. Higher A-values indicate a stronger preference for the equatorial position. This is a significant concept for understanding the conformational analysis of substituted cyclohexanes.
-
Predicting Conformational Preference: By knowing the A-values of different substituents, you can accurately predict the preferred conformation of substituted cyclohexanes. This is crucial in predicting reactivity and physical properties.
Boat Conformation: A Less Stable Alternative
Another conformation of cyclohexane is the boat conformation. In this structure, the ring adopts a more "boat-like" shape. However, the boat conformation is significantly less stable than the chair conformation due to several factors:
-
Flagpole Interactions: Two hydrogen atoms, termed "flagpole" hydrogens, are brought into close proximity in the boat conformation, leading to strong steric repulsion.
-
Torsional Strain: Some bonds in the boat conformation experience eclipsing interactions, also contributing to its higher energy.
-
Twist-Boat Conformation: A slightly modified boat conformation, the twist-boat, mitigates some of the steric strain present in the standard boat conformation. Although still less stable than the chair, it's more stable than a simple boat conformation.
Other Conformations and Interconversions:
While the chair and boat conformations are the most commonly discussed, other less stable conformations exist. The cyclohexane molecule undergoes rapid interconversion between different conformations at room temperature, a process known as ring flipping.
Ring Flipping:
Ring flipping is an important dynamic process in cyclohexane's behavior. This process involves the transition between two chair conformations, passing through a high-energy transition state.
-
Mechanism: The ring flipping mechanism involves several bond rotations and changes in dihedral angles. Visualizing this process with molecular models is very helpful for understanding this fundamental transformation.
-
Equilibration: The interconversion between chair conformations reaches equilibrium, with the most stable conformation predominating. This equilibrium is influenced by the nature and position of any substituents present on the ring.
Substituted Cyclohexanes: Conformational Analysis
The presence of substituents on the cyclohexane ring adds another layer of complexity to conformational analysis. The size and nature of the substituent significantly impact the relative stability of different conformations.
Monosubstituted Cyclohexanes:
A single substituent on cyclohexane will favor the equatorial position in most cases. This is due to the minimization of steric interactions with other atoms on the ring.
Disubstituted Cyclohexanes:
The conformational analysis of disubstituted cyclohexanes becomes more intricate. The relative positions of the substituents (cis or trans) determine the relative stability of the possible chair conformations.
-
Cis Isomers: Cis isomers have substituents on the same side of the ring. In cis isomers, one chair conformation will have both substituents axial and the other equatorial.
-
Trans Isomers: Trans isomers have substituents on opposite sides of the ring. In the trans isomer, one of the chair conformations will have both substituents equatorial and the other axial.
-
Predicting Stability: For disubstituted cyclohexanes, the most stable conformation minimizes the number of axial substituents. This is the basis for understanding their chemical and physical properties.
Applications and Importance:
Understanding the 3D structure and conformations of cyclohexane is crucial in several areas of chemistry and beyond:
-
Organic Chemistry: Cyclohexane serves as a foundational model for understanding conformational analysis in larger, more complex molecules. This understanding is essential for designing and synthesizing new molecules with specific properties.
-
Medicinal Chemistry: Many drugs contain cyclohexane rings. Understanding cyclohexane's conformations is critical for optimizing drug design and efficacy. The conformation of a drug molecule can significantly influence its interactions with biological targets.
-
Polymer Chemistry: Cyclohexane derivatives are used in the synthesis of polymers. The conformation of the cyclohexane unit within the polymer chain affects the overall properties of the material.
-
Material Science: Cyclohexane and its derivatives are used in various materials such as solvents, plastics, and fibers. Understanding their structure-property relationships is important for designing materials with specific functionalities.
-
Spectroscopy: Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful technique used to determine the conformation of molecules. Analyzing the NMR spectrum of cyclohexane provides valuable information on its conformational dynamics.
Conclusion: A Deeper Dive into Cyclohexane's 3D World
The three-dimensional representation of cyclohexane is far more than a simple molecular model; it's a gateway to understanding fundamental principles of organic chemistry, including conformational analysis, steric strain, and the interplay between structure and properties. By exploring its various conformations, their relative stabilities, and their influence on chemical reactivity, we gain a deeper appreciation for the complexity and beauty of this seemingly simple molecule. The applications of this knowledge extend far beyond the realm of organic chemistry, impacting diverse fields such as medicinal chemistry, polymer science, and materials science. Continuing research in these areas will undoubtedly reveal further insights into the fascinating world of cyclohexane and its derivatives.
Latest Posts
Latest Posts
-
John Is Rollerblading Down A Long
Mar 19, 2025
-
Hydrolysis Of Sucrose A Disaccharide Results In
Mar 19, 2025
-
What Is The Difference Between Mutualism And Synergism
Mar 19, 2025
-
The Supply Of A Good Will Be More Elastic The
Mar 19, 2025
-
What Do Economists Mean When They Say Behavior Is Rational
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about A 3d Representation Of A Cyclohexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
